Exploring TMPRSS2 Drug Target to Combat Influenza and Coronavirus Infection
et al., Scientifica, doi:10.1155/sci5/3687892, Jan 2025
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
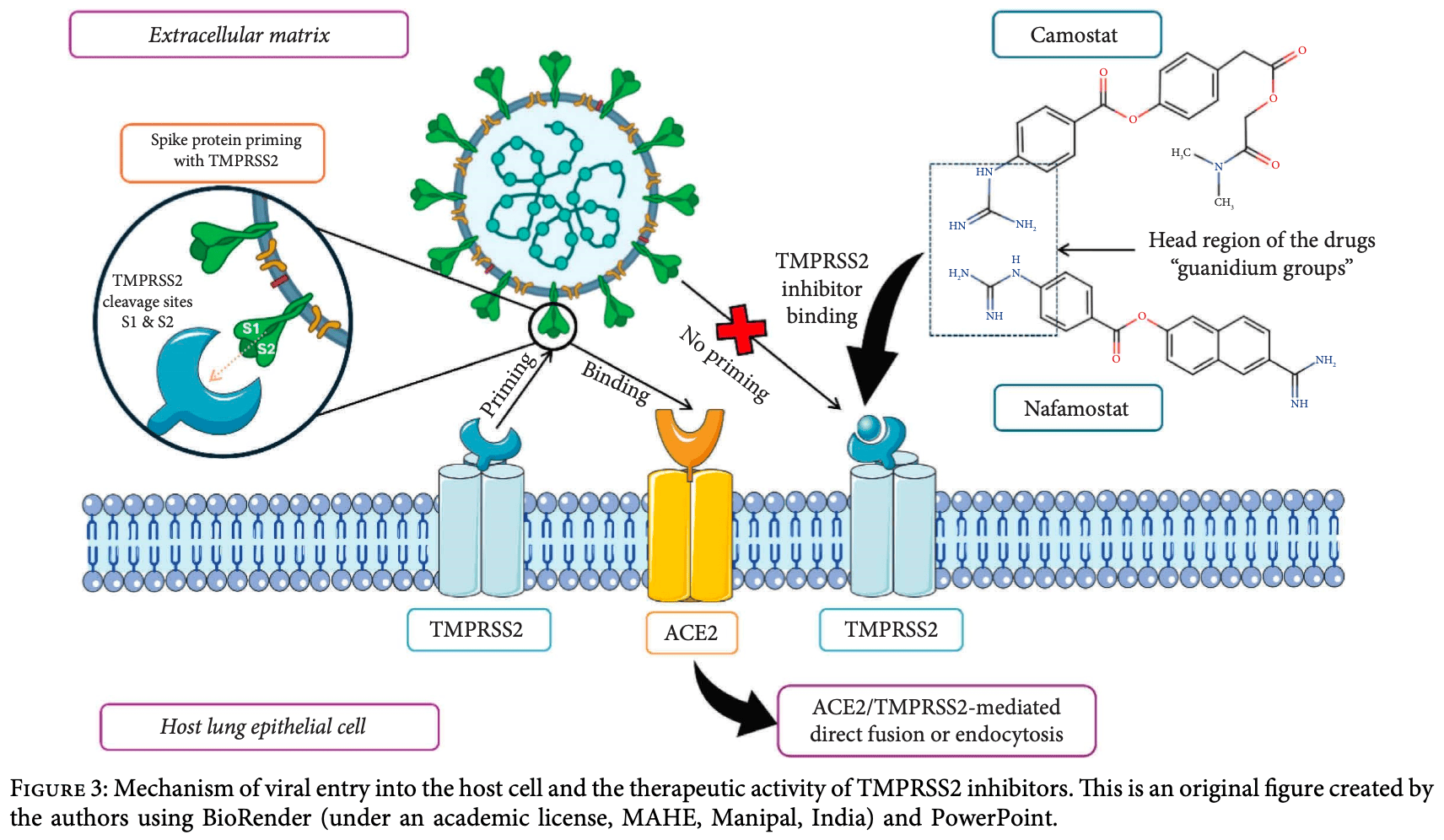
Review of TMPRSS2 as a drug target to combat influenza and coronavirus infections. Authors highlight that TMPRSS2, a transmembrane serine protease coexpressed with ACE2 receptors in the human respiratory tract, plays a critical role in viral pathogenesis by cleaving and activating viral surface proteins. For SARS-CoV-2, TMPRSS2 cleaves the spike protein to facilitate membrane fusion and viral entry, while for influenza viruses, it activates the hemagglutinin protein essential for viral spread. Authors detail how TMPRSS2 inhibitors can reduce viral propagation and disease severity by blocking viral entry into respiratory cells. Several TMPRSS2 inhibitors including camostat mesylate, nafamostat, MM3122, and DRP-104 have shown promise in preclinical studies. Authors explore the structure-function relationship of TMPRSS2, its tissue distribution, mechanisms of viral protein activation, and potential therapeutic strategies for inhibiting it.
1.
Saha et al., Inhaled Dry Powder of Antiviral Agents: A Promising Approach to Treating Respiratory Viral Pathogens, Viruses, doi:10.3390/v17020252.
2.
Baby et al., Exploring TMPRSS2 Drug Target to Combat Influenza and Coronavirus Infection, Scientifica, doi:10.1155/sci5/3687892.
3.
Mitev, V., Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib, Pharmacia, doi:10.3897/pharmacia.70.e112550.
Baby et al., 31 Jan 2025, India, peer-reviewed, 7 authors.
Contact: yogendra.nayak@manipal.edu.
Exploring TMPRSS2 Drug Target to Combat Influenza and Coronavirus Infection
Scientifica, doi:10.1155/sci5/3687892
Respiratory viral infections, including infuenza and coronaviruses, present signifcant health risks worldwide. Te recent COVID-19 pandemic highlights the urgent need for novel and efective antiviral agents. Te host cell protease, transmembrane serine protease 2 (TMPRSS2), facilitates viral pathogenesis by playing a critical role in viral invasion and disease progression. Tis protease is coexpressed with the viral receptors of angiotensin-converting enzyme 2 (ACE2) for SARS-CoV-2 in the human respiratory tract and plays a signifcant role in activating viral proteins and spreading. TMPRSS2 activates the coronavirus spike (S) protein and permits membrane fusion and viral entry by cleaving the virus surface glycoproteins. It also activates the hemagglutinin (HA) protein, an enzyme necessary for the spread of infuenza virus. TMPRSS2 inhibitors can reduce viral propagation and morbidity by blocking viral entry into respiratory cells and reducing viral spread, infammation, and disease severity. Tis review examines the role of TMPRSS2 in viral replication and pathogenicity. It also ofers potential avenues to develop targeted antivirals to inhibit TMPRSS2 function, suggesting a possible focus on targeted antiviral development. Ultimately, the review seeks to contribute to improving public health outcomes related to these viral infections.
Conflicts of Interest Te authors declare no conficts of interest.
References
Abdollahi, Izadi, TMPRSS2 as an Infuential Human Gene for COVID-19, Journal of Human Genetics and Genomics, doi:10.5812/jhgg.119384
Abiyyi, Dwira, Bustami, Erlina, Terapeutic Options for COVID-19: Drug Repurposing of Serine Protease Inhibitor Against TMPRSS2, Indonesian Journal of Medical Chemistry and Bioinformatics, doi:10.7454/ijmcb.v1i2.1001
Ahmed, Anam, Ahmed, Development of Galectin-3 Targeting Drugs for Terapeutic Applications in Various Diseases, International Journal of Molecular Sciences, doi:10.3390/ijms24098116
Alhumaid, Alabdulqader, Dossary, Global Coinfections With Bacteria, Fungi, and Respiratory Viruses in Children With SARS-CoV-2: A Systematic Review and Meta-Analysis, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed7110380
Amani, Khanijahani, Amani, Hydroxychloroquine Plus Standard of Care Compared with Standard of Care Alone in COVID-19: A Meta-Analysis of Randomized Controlled Trials, Scientifc Reports, doi:10.1038/s41598-021-91089-3
Arabi, Fowler, Hayden, Critical Care Management of Adults With Community-Acquired Severe Respiratory Viral Infection, Intensive Care Medicine, doi:10.1007/s00134-020-05943-5
Aviani, Halim, Soeroto, Achmad, Djuwantono, Current Views on the Potentials of Convalescent Plasma Terapy (CPT) as Coronavirus Disease 2019 (COVID-19) Treatment: A Systematic Review and Meta-Analysis Based on Recent Studies and Previous Respiratory Pandemics, Reviews in Medical Virology, doi:10.1002/rmv.2225
Baby, Maity, Mehta, Suresh, Nayak et al., SARS-CoV-2 Entry Inhibitors by Dual Targeting TMPRSS2 and ACE2: An In Silico Drug Repurposing Study, European Journal of Pharmacology, doi:10.1016/j.ejphar.2021.173922
Baden, El Sahly, Essink, Efcacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, New England Journal of Medicine, doi:10.1056/NEJMoa2035389
Behzadi, Leyva-Grado, Overview of Current Terapeutics and Novel Candidates against Infuenza, Respiratory Syncytial Virus, and Middle East Respiratory Syndrome Coronavirus Infections, Frontiers in Microbiology, doi:10.3389/fmicb.2019.01327
Bernal, Gomes Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine, doi:10.1056/NEJMoa2116044
Bertram, Dijkman, Habjan, TMPRSS2 Activates the Human Coronavirus 229E for Cathepsin-Independent Host Cell Entry and Is Expressed in Viral Target Cells in the Respiratory Epithelium, Journal of Virology, doi:10.1128/jvi.03372-12
Bertram, Heurich, Lavender, Infuenza and SARS-Coronavirus Activating Proteases TMPRSS2 and HAT Are Expressed at Multiple Sites in Human Respiratory and Gastrointestinal Tracts, PLoS One, doi:10.1371/journal.pone.0035876
Bestle, Heindl, Limburg, TMPRSS2 and Furin are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells, Life Science Alliance, doi:10.26508/lsa.202000786
Bestle, Limburg, Kruhl, Hemagglutinins of Avian Infuenza Viruses Are Proteolytically Activated by TMPRSS2 in Human and Murine Airway Cells, Journal of Virology, doi:10.1128/JVI.00906-21
Boon, Bricker, Fritch, Efcacy of Host Cell Serine Protease Inhibitor MM3122 Against SARS-CoV-2 for Treatment and Prevention of COVID-19, Journal of Virology, doi:10.1128/jvi.01903-23
Böttcher-Friebertshäuser, Freuer, Sielaf, Cleavage of Infuenza Virus Hemagglutinin by Airway Proteases TMPRSS2 and HAT Difers in Subcellular Localization and Susceptibility to Protease Inhibitors, Journal of Virology, doi:10.1128/jvi.00140-10
Böttcher-Friebertshäuser, Lu, Meyer, Hemagglutinin Activating Host Cell Proteases Provide Promising Drug Targets for the Treatment of Infuenza A and B Virus Infections, Vaccine, doi:10.1016/j.vaccine.2012.10.001
Böttcher-Friebertshäuser, Stein, Klenk, Garten, Inhibition of Infuenza Virus Infection in Human Airway Cell Cultures by an Antisense Peptide-Conjugated Morpholino Oligomer Targeting the Hemagglutinin-Activating Protease TMPRSS2, Journal of Virology, doi:10.1128/jvi.01294-10
Caniglia, Asuthkar, Tsung, Guda, Velpula, Immunopathology of Galectin-3: an Increasingly Promising Target in COVID-19, F1000Res, doi:10.12688/f1000research.25979.1
Cao, Feng, Wang, Computational Analysis of TMPRSS2 Expression in Normal and SARS-CoV-2-Infected Human Tissues, Chemico-Biological Interactions, doi:10.1016/j.cbi.2021.109583
Cates, Lucero-Obusan, Dahl, Risk for In-Hospital Complications Associated With COVID-19 and Infuenza-Veterans Health Administration, United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6942e3
Chan, Zhan, Te Emergence of the Spike Furin Cleavage Site in SARS-CoV-2, Molecular Biology and Evolution, doi:10.1093/molbev/msab327
Chanawong, Mackenzie, Mckinnon, Hu, Meech, Exemestane and Its Active Metabolite 17-Hydroexemestane Induce UDP-Glucuronosyltransferase (UGT) 2B17 Expression in Breast Cancer Cells, Journal of Pharmacology and Experimental Terapeutics, doi:10.1124/jpet.117.240317
Chen, Lee, Lucht, TMPRSS2, a Serine Protease Expressed in the Prostate on the Apical Surface of Luminal Epithelial Cells and Released into Semen in Prostasomes, Is Misregulated in Prostate Cancer Cells, American Journal Of Pathology, doi:10.2353/ajpath.2010.090665
Chen, Lin, Chen, A Multicenter, Randomized, Open-Label, Controlled Trial to Evaluate the Effcacy and Tolerability of Hydroxychloroquine and a Retrospective Study in Adult Patients With Mild to Moderate Coronavirus Disease 2019 (COVID-19), PLoS One, doi:10.1371/journal.pone.0242763
Cheng, Chao, Li, Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Efects, Cell Reports, doi:10.1016/j.celrep.2020.108254
Cheng, Zhou, To, Identifcation of TMPRSS2 as a Susceptibility Gene for Severe 2009 Pandemic A(H1N1) Infuenza and A(H7N9) Infuenza, Journal of Infectious Diseases, doi:10.1093/infdis/jiv246
Choi, Ren, Chen, Exchange Proteins Directly Activated by cAMP and Teir Roles in Respiratory Syncytial Virus Infection, Journal of Virology, doi:10.1128/jvi.01200-18
Choi, Shin, Kang, Park, Beck, Target-Centered Drug Repurposing Predictions of Human Angiotensin-Converting Enzyme 2 (ACE2) and Transmembrane Protease Serine Subtype 2 (TMPRSS2) Interacting Approved Drugs for Coronavirus Disease 2019 (COVID-19) Treatment Trough a Drug-Target Interaction Deep Learning Model, Viruses, doi:10.3390/v12111325
Choi, Wu, Cong, Broad Impact of Exchange Protein Directly Activated by Camp 2 (Epac2) on Respiratory Viral Infections, Viruses, doi:10.3390/v13061179
Choudhary, Silakari, Scafold Morphing of Arbidol (Umifenovir) in Search of Multi-Targeting Terapy Halting the Interaction of SARS-CoV-2 With ACE2 and Other Proteases Involved in COVID-19, Virus Research, doi:10.1016/j.virusres.2020.198146
Ciacci Zanella, Snyder, Arruda, Pigs Lacking TMPRSS2 Displayed Fewer Lung Lesions and Reduced Infammatory Response When Infected With Infuenza A Virus, Frontiers in Genome Editing, doi:10.3389/fgeed.2023.1320180
Citarella, Dimasi, Moi, Recent Advances in SARS-CoV-2 Main Protease Inhibitors: From Nirmatrelvir to Future Perspectives, Biomolecules, doi:10.3390/biom13091339
Damasio, Pereira, Moreira, Duarte Dos Santos, Dalla-Costa et al., Does Virus-Bacteria Coinfection Increase the Clinical Severity of Acute Respiratory Infection?, Journal of Medical Virology, doi:10.1002/jmv.24210
Di Maio, Scutari, Forqué, Presence and Signifcance of Multiple Respiratory Viral Infections in Children Admitted to a Tertiary Pediatric Hospital in Italy, Viruses, doi:10.3390/v16050750
Eastman, Roth, Brimacombe, Correction to Remdesivir: A Review of Its Discovery and Development Leading to Human Clinical Trials for Treatment of COVID-19, ACS Central Science, doi:10.1021/acscentsci.0c00747
Elbadwi, Khairy, Alsamani, Identifcation of Novel Transmembrane Protease Serine Type 2 Drug Candidates for COVID-19 Using Computational Studies, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2021.100725
Elmezayen, Al-Obaidi, Yelekçi, Drug Repurposing for Coronavirus (COVID-19): In Silico Screening of Known Drugs Against Coronavirus 3CL Hydrolase and Protease Enzymes, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1758791
Essalmani, Jain, Susan-Resiga, Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity, Journal of Virology, doi:10.1128/jvi.00128-22
Falsey, Mcelhaney, Beran, Respiratory Syncytial Virus and Other Respiratory Viral Infections in Older Adults With Moderate to Severe Infuenza-Like Illness, Te Journal of Infectious Diseases, doi:10.1093/infdis/jit839
Focosi, Casadevall, Franchini, Maggi, Sotrovimab: A Review of Its Efcacy Against SARS-CoV-2 Variants, Viruses, doi:10.3390/v16020217
Fraser, Beldar, Seitova, Structure and Activity of Human TMPRSS2 Protease Implicated in SARS-CoV-2 Activation, Nature Chemical Biology, doi:10.1038/s41589-022-01059-7
Gallo, Arienzo, Iacobelli, Iacobelli, Antonini, Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2, International Journal of Molecular Sciences, doi:10.3390/ijms23137314
Galloway, Reed, Russell, Steinhauer, Infuenza HA Subtypes Demonstrate Divergent Phenotypes for Cleavage Activation and pH of Fusion: Implications for Host Range and Adaptation, PLoS Pathogens, doi:10.1371/journal.ppat.1003151
Gamba, Van Eijk, Lányi, PK/PD Investigation of Antiviral Host Matriptase/TMPRSS2 Inhibitors in Cell Models, Scientifc Reports, doi:10.1038/s41598-024-67633-2
Gi, Virtual Drug Repurposing Study Against SARS-CoV-2 TMPRSS2 Target, Turkish Journal of Biology, doi:10.3906/biy-2005-112
Gierer, Bertram, Kaup, Te Spike Protein of the Emerging Betacoronavirus EMC Uses a Novel Coronavirus Receptor for Entry, Can Be Activated by TMPRSS2, and Is Targeted by Neutralizing Antibodies, Journal of Virology, doi:10.1128/JVI.00128-13
Glowacka, Bertram, Müller, Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response, Journal of Virology, doi:10.1128/jvi.02232-10
Grisard, Schörner, Barazzetti, ACE2 and TMPRSS2 Expression in Patients Before, During, and After SARS-CoV-2 Infection, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2024.1355809
Grohskopf, Blanton, Ferdinands, Prevention and Control of Seasonal Infuenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2022-23 Infuenza Season, MMWR. Recommendations and Reports, doi:10.15585/mmwr.rr7101a1
Gunst, Staerke, Pahus, Efcacy of the TMPRSS2 Inhibitor Camostat Mesilate in Patients Hospitalized With Covid-19-A Double-Blind Randomized Controlled Trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100849
Guo, Porter, Crozier, Topical TMPRSS2 Inhibition Prevents SARS-CoV-2 Infection in Diferentiated Human Airway Cultures, Life Science Alliance, doi:10.26508/lsa.202101116
Harbig, Mernberger, Bittel, Transcriptome Profling and Protease Inhibition Experiments Identify Proteases Tat Activate H3N2 Infuenza A and Infuenza B Viruses in Murine Airways, Journal of Biological Chemistry, doi:10.1074/jbc.RA120.012635
Hashimoto, Sakamoto, Deguchi, Dual Inhibition of TMPRSS2 and Cathepsin B Prevents SARS-CoV-2 Infection in iPS Cells, Molecular Terapy-Nucleic Acids, doi:10.1016/j.omtn.2021.10.016
Hatesuer, Bertram, Mehnert, Tmprss2 Is Essential for Infuenza H1N1 Virus Pathogenesis in Mice, PLoS Pathogens, doi:10.1371/journal.ppat.1003774
Hayden, Sugaya, Hirotsu, Baloxavir Marboxil for Uncomplicated Infuenza in Adults and Adolescents, New England Journal of Medicine, doi:10.1056/NEJMoa1716197
Heindl, Rupp, Schwerdtner, ACE2 Acts as a Novel Regulator of TMPRSS2-Catalyzed Proteolytic Activation of Infuenza A Virus in Airway Cells, Journal of Virology, doi:10.1128/jvi.00102-24
Heneghan, Onakpoya, Tompson, Spencer, Jones et al., Zanamivir for Infuenza in Adults and Children: Systematic Review of Clinical Study Reports and Summary of Regulatory Comments, BMJ, doi:10.1136/bmj.g2547
Hernandez, Roman, Pasupuleti, Barboza, White, Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19: A Living Systematic Review, Annals of Internal Medicine, doi:10.7326/M20-2496
Heurich, Hofmann-Winkler, Gierer, Liepold, Jahn et al., TMPRSS2 and ADAM17 Cleave ACE2 Diferentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein, Journal of Virology, doi:10.1128/jvi.02202-13
Hofmann, Hofmann-Winkler, Smith, Camostat Mesylate Inhibits SARS-CoV-2 Activation by TMPRSS2-Related Proteases and Its Metabolite GBPA Exerts Antiviral Activity, EBioMedicine, doi:10.1016/j.ebiom.2021.103255
Hofmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hoy, Abiraterone Acetate: A Review of its Use in Patients With Metastatic Castration-Resistant Prostate Cancer, Drugs, doi:10.1007/s40265-013-0150-z
Hu, Shrimp, Guo, Discovery of TMPRSS2 Inhibitors From Virtual Screening as a Potential Treatment of COVID-19, ACS Pharmacology & Translational Science, doi:10.1021/acsptsci.0c00221
Huggins, Structural Analysis of Experimental Drugs Binding to the SARS-CoV-2 Target TMPRSS2, Journal of Molecular Graphics and Modelling, doi:10.1016/j.jmgm.2020.107710
Iwata-Yoshikawa, Kakizaki, Shiwa-Sudo, Essential Role of TMPRSS2 in SARS-CoV-2 Infection in Murine Airways, Nature Communications, doi:10.1038/s41467-022-33911-8
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection, Journal of Virology, doi:10.1128/jvi.01815-18
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 Entry Into Cells, Nature Reviews Molecular Cell Biology, doi:10.1038/s41580-021-00418-x
Jean, Lee, Hsueh, Treatment Options for COVID-19: Te Reality and Challenges, Journal of Microbiology, Immunology, and Infection, doi:10.1016/j.jmii.2020.03.034
Jeferson, Jones, Doshi, Spencer, Onakpoya et al., Oseltamivir for Infuenza in Adults and Children: Systematic Review of Clinical Study Reports and Summary of Regulatory Comments, BMJ, doi:10.1136/bmj.g2545
Jiang, Ouyang, Yin, Proxalutamide in Patients With AR-Positive Metastatic Breast Cancer: Results From an Open-Label Multicentre Phase Ib Study and 24 Scientifca 6168, European Journal of Cancer, doi:10.1016/j.ejca.2022.08.025
Karolyi, Pawelka, Omid, Camostat Mesylate versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19-Results from a Randomized, Controlled, Open Label, Platform Trial (ACOVACT), Frontiers in Pharmacology, doi:10.3389/fphar.2022.870493
Kashour, Kashour, Gerberi, Tleyjeh, Mortality, Viral Clearance, and Other Clinical Outcomes of Hydroxychloroquine in COVID-19 Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Clinical and Translational Science, doi:10.1111/cts.13001
Kazuya, Kazuhiko, Miyuki, Shutoku, Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry, Journal of Virology, doi:10.1128/jvi.01387-16
Kehdy, Pita-Oliveira, Scudeler, Human-SARS-CoV-2 Interactome and Human Genetic Diversity: TMPRSS2-Rs2070788, Associated With Severe Infuenza, and Its Population Genetics Caveats in Native Americans, Genetics and Molecular Biology, doi:10.1590/1678-4685-gmb-2020-0484
Keller, Böttcher-Friebertshäuser, Lohof, TMPRSS2, a Novel Host-Directed Drug Target Against SARS-CoV-2, Signal Transduction and Targeted Terapy, doi:10.1038/s41392-022-01084-x
Khoury, Cuenca, Cruz, Figueroa, Rocco et al., Current Status of Cell-Based Terapies for Respiratory Virus Infections: Applicability to COVID-19, European Respiratory Journal, doi:10.1183/13993003.00858-2020
Koch, Uckeley, Doldan, Stanifer, Boulant et al., TMPRSS2 Expression Dictates the Entry Route Used by SARS-CoV-2 to Infect Host Cells, Te EMBO Journal, doi:10.15252/embj.2021107821
Kühn, Bergmann, Kösterke, Te Proteolytic Activation of (H3N2) Infuenza A Virus Hemagglutinin Is Facilitated by Diferent Type II Transmembrane Serine Proteases, Journal of Virology, doi:10.1128/JVI.02693-15
Lambertz, Gerhauser, Nehlmeier, Tmprss2 Knock-Out Mice are Resistant to H10 Infuenza A Virus Pathogenesis, Journal of General Virology, doi:10.1099/jgv.0.001274
Lavie, Dubuisson, Belouzard, SARS-CoV-2 Spike Furin Cleavage Site and S2′ Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner, Journal of Virology, doi:10.1128/jvi.00474-22
Li, Pillai, Miyake, Nair, Te Role of Viral Co-Infections in the Severity of Acute Respiratory Infections Among Children Infected With Respiratory Syncytial Virus (RSV): A Systematic Review and Meta-Analysis, Journal of Global Health, doi:10.7189/JOGH.10.010426
Lie, Synowiec, Mazur, Rabalski, Pyrć, An Engineered A549 Cell Line Expressing CD13 and TMPRSS2 Is Permissive to Clinical Isolate of Human Coronavirus 229E, Virology, doi:10.1016/j.virol.2023.109889
Limburg, Harbig, Bestle, TMPRSS2 Is the Major Activating Protease of Infuenza A Virus in Primary Human Airway Cells and Infuenza B Virus in Human Type II Pneumocytes, Journal of Virology, doi:10.1128/JVI.00649-19
Liu, Huang, Smits, Human-Type Sialic Acid Receptors Contribute to Avian Infuenza A Virus Binding and Entry by Hetero-Multivalent Interactions, Nature Communications, doi:10.1038/s41467-022-31840-0
Liu, Qu, Qu, Tian, Hakonarson, Expression Pattern of the SARS-CoV-2 Entry Genes ACE2 and TMPRSS2 in the Respiratory Tract, Viruses, doi:10.3390/v12101174
Liu, Shahed-Al-Mahmud, Chen, A Carbohydrate-Binding Protein From the Edible Lablab Beans Efectively Blocks the Infections of Infuenza Viruses and SARS-CoV-2, Cell Reports, doi:10.1016/j.celrep.2020.108016
Lu, Liu, Selective Estrogen Receptor Degraders (SERDs): A Promising Strategy for Estrogen Receptor Positive Endocrine-Resistant Breast Cancer, Journal of Medicinal Chemistry, doi:10.1021/acs.jmedchem.0c00913
Lubinski, Whittaker, Te SARS-CoV-2 Furin Cleavage Site: Natural Selection or Smoking Gun?, Te Lancet Microbe, doi:10.1016/S2666-5247(23)00144-1
Lucas, Heinlein, Kim, Te Androgen-Regulated Protease TMPRSS2 Activates a Proteolytic Cascade Involving Components of the Tumor Microenvironment and Promotes Prostate Cancer Metastasis, Cancer Discovery, doi:10.1158/2159-8290.CD-13-1010
Lukassen, Chua, Trefzer, SARS-CoV-2 Receptor ACE2 and TMPRSS2 Are Primarily Expressed in Bronchial Transient Secretory Cells, Te EMBO Journal, doi:10.15252/embj.20105114
Lü, Qiu, Jia, Guo, Leng, Single-cell Expression Profles of ACE2 and TMPRSS2 Reveals Potential Vertical Transmission and Fetus Infection of SARS-CoV-2, Aging, doi:10.18632/aging.104015
Mahoney, Damalanka, Tartell, A Novel Class of TMPRSS2 Inhibitors Potently Block SARS-CoV-2 and MERS-CoV Viral Entry and Protect Human Epithelial Lung Cells, Proceedings of the National Academy of Sciences of the United States of America, doi:10.1073/pnas.2108728118
Mantzourani, Vasilakaki, Gerogianni, Kokotos, Te Discovery and Development of Transmembrane Serine Protease 2 (TMPRSS2) Inhibitors as Candidate Drugs for the Treatment of COVID-19, Expert Opinion on Drug Discovery, doi:10.1080/17460441.2022.2029843
Marín-Aguilera, Reig, Milà-Guasch, Te Infuence of Treatment Sequence in the Prognostic Value of TMPRSS2-ERG as Biomarker of Taxane Resistance in Castration-resistant Prostate Cancer, International Journal of Cancer, doi:10.1002/ijc.32238
Matsuyama, Nagata, Shirato, Kawase, Takeda et al., Efcient Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by the Transmembrane Protease TMPRSS2, Journal of Virology, doi:10.1128/jvi.01542-10
Matsuyama, Nao, Shirato, Enhanced Isolation of SARS-CoV-2 by TMPRSS2-Expressing Cells, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2002589117
Meng, Abdullahi, Ferreira, Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity, Nature, doi:10.1038/s41586-022-04474-x
Meng, Gao, Liu, Ginsenosides, Potential TMPRSS2 Inhibitors, a Trade-Of Between the Terapeutic Combination for Anti-PD-1 Immunotherapy and the Treatment of COVID-19 Infection of LUAD Patients, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1085509
Metzdorf, Jacobsen, Greweling-Pils, TMPRSS2 Is Essential for SARS-CoV-2 Beta and Omicron Infection, Viruses, doi:10.3390/v15020271
Meyer, Sielaf, Hammami, Böttcher-Friebertshäuser, Garten et al., Identifcation of the First Synthetic Inhibitors of the Type II Transmembrane Serine Protease TMPRSS2 Suitable for Inhibition of Infuenza Virus Activation, Biochemical Journal, doi:10.1042/BJ20130101
Mochan, Sego, Mathematical Modeling of the Lethal Synergism of Coinfecting Pathogens in Respiratory Viral Infections: A Review, Microorganisms, doi:10.3390/microorganisms11122974
Mukherjee, Wanjari, Nagarajan, Letrozole: Pharmacology, Toxicity and Potential Terapeutic Efects, Life Sciences, doi:10.1016/j.lfs.2022.121074
Muus, Luecken, Eraslan, Single-Cell Meta-Analysis of SARS-CoV-2 Entry Genes Across Tissues and Demographics, Nature Medicine, doi:10.1038/s41591-020-01227-z
Nazerian, Vakili, Ebrahimi, Niknejad, Developing Cytokine Storm-Sensitive Terapeutic Strategy in COVID-19 Using 8P9R Chimeric Peptide and Soluble ACE2, Frontiers in Cell and Developmental Biology, doi:10.3389/fcell.2021.717587
Oduro-Kwateng, Soliman, DON/DRP-104 as Potent Serine Protease Inhibitors Implicated in SARS-CoV-2 Infection: Comparative Binding Modes With Human TMPRSS2 and Novel Terapeutic Approach, Journal of Cellular Biochemistry, doi:10.1002/jcb.30528
Padmanabhan, Desikan, Dixit, Targeting TMPRSS2 and Cathepsin B/L Together May Be Synergistic Against SARS-CoV-2 Infection, PLoS Computational Biology, doi:10.1371/journal.pcbi.1008461
Palit, Chattopadhyay, Tomas, Kundu, Kim et al., Phytopharmaceuticals Mediated Furin and TMPRSS2 Receptor Blocking: Can It Be a Potential Terapeutic Option for Covid-19?, Phytomedicine, doi:10.1016/j.phymed.2020.153396
Paniri, Hosseini, Akhavan-Niaki, First Comprehensive Computational Analysis of Functional Consequences of TMPRSS2 SNPs in Susceptibility to SARS-CoV-2 Among Diferent Populations, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1767690
Paoloni-Giacobino, Chen, Peitsch, Rossier, Antonarakis, Cloning of the TMPRSS2 Gene, Which Encodes a Novel Serine Protease With Transmembrane, LDLRA, and SRCR Domains and Maps to 21q22.3, Genomics, doi:10.1006/geno.1997.4845
Peacock, Goldhill, Zhou, Te Furin Cleavage Site in the SARS-CoV-2 Spike Protein Is Required for Transmission in Ferrets, Nat Microbiol, doi:10.1038/s41564-021-00908-w
Peters, Sajuthi, Deford, COVID-19-Related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids, American Journal of Respiratory and Critical Care Medicine, doi:10.1164/rccm.202003-0821OC
Polack, Tomas, Kitchin, Safety and Efcacy of the BNT162b2 mRNA Covid-19 Vaccine, New England Journal of Medicine, doi:10.1056/NEJMoa2034577
Qiao, Wang, Mannan, Targeting Transcriptional Regulation of SARS-CoV-2 Entry Factors ACE2 and TMPRSS2, Proceedings of the National Academy of Sciences of the United States of America, doi:10.1073/pnas.2021450118
Qiao, Wotring, Zheng, Proxalutamide Reduces SARS-CoV-2 Infection and Associated Infammatory Response, Proceedings of the National Academy of Sciences of the United States of America, doi:10.1073/pnas.2221809120
Reuter, Chen, Kropf, SARS-CoV-2 Spike Protein Is Capable of Inducing Cell-Cell Fusions Independent From Its Receptor ACE2 and Tis Activity Can Be Impaired by Furin Inhibitors or a Subset of Monoclonal Antibodies, Viruses, doi:10.3390/v15071500
Robinson, Busl, Neurologic Manifestations of Severe Respiratory Viral Contagions, Critical Care Explorations, doi:10.1097/cce.0000000000000107
Romeu, Probable Human Origin of the SARS-CoV-2 Polybasic Furin Cleavage Motif, BMC Genom Data, doi:10.1186/s12863-023-01169-8
Ryabkova, Churilov, Shoenfeld, Infuenza Infection, SARS, MERS and COVID-19: Cytokine Storm-the Common Denominator and the Lessons to Be Learned, Clinical Immunology, doi:10.1016/J.CLIM.2020.108652
Réa-Neto, Bernardelli, Câmara, Reese, Queiroga et al., An Open-Label Randomized Controlled Trial Evaluating the Efcacy of Chloroquine/Hydroxychloroquine in Severe COVID-19 Patients, Scientifc Reports, doi:10.1038/s41598-021-88509-9
Sakai, Ami, Nakajima, TMPRSS2 Independency for Haemagglutinin Cleavage In Vivo Diferentiates Infuenza B Virus From Infuenza A Virus, Scientifc Reports, doi:10.1038/srep29430
Sakai, Ami, Tahara, Te Host Protease TMPRSS2 Plays a Major Role in In Vivo Replication of Emerging H7N9 and Seasonal Infuenza Viruses, Journal of Virology, doi:10.1128/jvi.03677-13
Samuel, Majd, Richter, Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated With Severe COVID-19 Symptoms in Men, Cell Stem Cell, doi:10.1016/j.stem.2020.11.009
Sarker, Das, Sarker, Roy, Momen, A Review on Expression, Pathological Roles, and Inhibition of TMPRSS2, the Serine Protease Responsible for SARS-CoV-2 Spike Protein Activation, Scientifca, doi:10.1155/2021/2706789
Schwerdtner, Schmacke, Nave, Unveiling the Role of TMPRSS2 in the Proteolytic Activation of Pandemic and Zoonotic Infuenza Viruses and Coronaviruses in Human Airway Cells, Viruses, doi:10.3390/v16111798
Schönfelder, Breuckmann, Elsner, Transmembrane Serine Protease 2 Polymorphisms and Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Type 2 Infection: A German Case-Control Study, Frontiers in Genetics, doi:10.3389/fgene.2021.667231
Scott, Peramivir: A Review in Uncomplicated Infuenza, Drugs, doi:10.1007/s40265-018-0981-8
Scotta, Chakr, De Moura, Respiratory Viral Coinfection and Disease Severity in Children: A Systematic Review and Meta-Analysis, Journal of Clinical Virology, doi:10.1016/j.jcv.2016.04.019
Self, Semler, Leither, Efect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19, JAMA, doi:10.1001/jama.2020.22240
Sgrignani, Cavalli, Computational Identifcation of a Putative Allosteric Binding Pocket in TMPRSS2, Frontiers in Molecular Biosciences, doi:10.3389/fmolb.2021.666626
Shakya, Chikhale, Bhat, Pharmacoinformatics-Based Identifcation of Transmembrane Protease Serine-2 Inhibitors From Morus Alba as SARS-CoV-2 Cell Entry Inhibitors, Molecular Diversity, doi:10.1007/s11030-021-10209-3
Shapira, Monreal, Dion, A TMPRSS2 Inhibitor Acts as a Pan-SARS-CoV-2 Prophylactic and Terapeutic, Nature, doi:10.1038/s41586-022-04661-w
Sharanya, Wilbee, Sathi, Natarajan, Computational Screening Combined With Well-Tempered Metadynamics Simulations Identifes Potential TMPRSS2 Inhibitors, Scientifc Reports, doi:10.1038/s41598-024-65296-7
Shen, Mao, Wu, Tanaka, Zhang, TMPRSS2: A Potential Target for Treatment of Infuenza Virus and Coronavirus Infections, Biochimie, doi:10.1016/j.biochi.2017.07.016
Shen, Qian, Yu, Inhibition of Infuenza A Virus Propagation by Benzoselenoxanthenes Stabilizing TMPRSS2 Gene G-Quadruplex and Hence Down-Regulating TMPRSS2 Expression, Scientifc Reports, doi:10.1038/s41598-020-64368-8
Shi, Arnott, Semogas, Te Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-Analysis, Te Journal of Infectious Diseases, doi:10.1093/infdis/jiy662
Shiraki, Daikoku, Favipiravir, an Anti-Infuenza Drug against Life-Treatening RNA Virus Infections, Pharmacology & Terapeutics, doi:10.1016/j.pharmthera.2020.107512
Sieben, Manley, Infuenza a Viruses Use Multivalent Sialic Acid Clusters for Cell Binding and Receptor Activation, Biophysical Journal, doi:10.1016/j.bpj.2015.11.2843
Simonovich, Burgos Pratx, Scibona, A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia, New England Journal of Medicine, doi:10.1056/NEJMoa2031304
Singh, Decroly, Khatib, Villoutreix, Structure-Based Drug Repositioning over the Human TMPRSS2 Protease Domain: Search for Chemical Probes Able to Repress SARS-CoV-2 Spike Protein Cleavages, European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2020.105495
Singh, O'reilly, Gewaid, Bowie, Gautier et al., Reactive Centre Loop Mutagenesis of Ser-pinB3 to Target TMPRSS2 and Furin: Inhibition of SARS-CoV-2 Cell Entry and Replication, International Journal of Molecular Sciences, doi:10.3390/ijms232012522
Stone, Frigault, Serling-Boyd, Efcacy of Tocilizumab in Patients Hospitalized With Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2028836
Stopsack, Mucci, Antonarakis, Nelson, Kantof, TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention?, Cancer Discovery, doi:10.1158/2159-8290.CD-20-0451
Strobelt, Adler, Shaul, Te Transmembrane Protease Serine 2 (TMPRSS2) Non-Protease Domains Regulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike-Mediated Virus Entry, Viruses, doi:10.3390/v15102124
Sun, Huang, Sun, Schizophyllum Commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2, International Journal of Molecular Sciences, doi:10.3390/IJMS232314766
Taingtamtanha, Baeurle, Study of Protease-Mediated Processes Initiating Viral Infection and Cell-Cell Viral Spreading of SARS-CoV-2, Journal of Molecular Modeling, doi:10.1007/s00894-022-05206-8
Takeda, Proteolytic Activation of SARS-CoV-2 Spike Protein, Microbiology and Immunology, doi:10.1111/1348-0421.12945
Tarnow, Engels, Arendt, TMPRSS2 Is a Host Factor that Is Essential for Pneumotropism and Pathogenicity of H7N9 Infuenza A Virus in Mice, Journal of Virology, doi:10.1128/JVI.03799-13
Tug, Fidan, Bozdayi, Te Relationship Between the Clinical Course of SARS-CoV-2 Infections and ACE2 and TMPRSS2 Expression and Polymorphisms, Advances in Clinical and Experimental Medicine, doi:10.17219/acem/163409
Tunders, Delahunt, Gene of the Month: TMPRSS2 (Transmembrane Serine Protease 2), Journal of Clinical Pathology, doi:10.1136/jclinpath-2020-206987
Ulrich, Troxel, Carmody, Treating COVID-19 with Hydroxychloroquine (TEACH): A Multicenter, Double-Blind Randomized Controlled Trial in Hospitalized Patients, Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa446
Vankadari, Ketavarapu, Mitnala, Vishnubotla, Reddy et al., Structure of Human TMPRSS2 in Complex With SARS-CoV-2 Spike Glycoprotein and Implications for Potential Terapeutics, Journal of Physical Chemistry Letters, doi:10.1021/acs.jpclett.2c00967
Vardhan, Sahoo, Virtual Screening by Targeting Proteolytic Sites of Furin and TMPRSS2 to Propose Potential Compounds Obstructing the Entry of SARS-CoV-2 Virus into Human Host Cells, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2021.04.001
Vink, Davis, Maclean, Viral Coinfections in Hospitalized Coronavirus Disease 2019 Patients Recruited to the International Severe Acute Respiratory and Emerging Infections Consortium WHO Clinical Characterisation Protocol UK Study, Open Forum Infectious Diseases, doi:10.1093/ofid/ofac531
Wettstein, Knaf, Kersten, Peptidomimetic Inhibitors of TMPRSS2 Block SARS-CoV-2 Infection in Cell Culture, Communications Biology, doi:10.1038/s42003-022-03613-4
Xia, Lan, Su, Te Role of Furin Cleavage Site in SARS-CoV-2 Spike Protein-Mediated Membrane Fusion in the Presence or Absence of Trypsin, Signal Transduction and Targeted Terapy, doi:10.1038/s41392-020-0184-0
Yaghoobi, Lord, Rezaiezadeh, Yekaninejad, Amini et al., TMPRSS2 Polymorphism (rs12329760) and the Severity of the COVID-19 in Iranian Population, PLoS One, doi:10.1371/journal.pone.0281750
Yousef, Valizadeh, Ghafari, Vahedi, Karbalaei et al., A Global Treatments for Coronaviruses Including COVID-19, Journal of Cellular Physiology, doi:10.1002/jcp.29785
Zarubin, Stepanov, Markov, Structural Variability, Expression Profle, and Pharmacogenetic Properties of TMPRSS2 Gene as a Potential Target for COVID-19 Terapy, Genes, doi:10.3390/genes12010019
Zeng, Langereis, Van Vliet, Huizinga, De Groot, Structure of Coronavirus Hemagglutinin-Esterase Ofers Insight Into Corona and Infuenza Virus Evolution, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.0800502105
Zhang, Kang, Gong, Digestive System Is a Potential Route of COVID-19: An Analysis of Single-Cell Coexpression Pattern of Key Proteins in Viral Entry Process, Gut, doi:10.1136/gutjnl-2020-320953
Zhang, Sun, Du, Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License Autoactivated Intracellularly and Requires N-Glycosylation for Regulation, Journal of Biological Chemistry, doi:10.1016/j.jbc.2022.102643
Zheng, Ma, Wang, Efcacy and Safety of Paxlovid for COVID-19: A Meta-Analysis, Journal of Infection, doi:10.1016/j.jinf.2022.09.027
Zhou, Wang, Liu, Disease Severity and Clinical Outcomes of Community-Acquired Pneumonia Caused by Non-Infuenza Respiratory Viruses in Adults: A Multicentre Prospective Registry Study From the CAP-China Network, European Respiratory Journal, doi:10.1183/13993003.02406-2018
Zmora, Blazejewska, Moldenhauer, DESC1 and MSPL Activate Infuenza A Viruses and Emerging Coronaviruses for Host Cell Entry, Journal of Virology, doi:10.1128/jvi.01427-14
Zmora, Hofmann, Kollmus, TMPRSS11A Activates the Infuenza A Virus Hemagglutinin and the MERS Coronavirus Spike Protein and Is Insensitive against Blockade by HAI-1, Journal of Biological Chemistry, doi:10.1074/jbc.RA118.001273
DOI record:
{
"DOI": "10.1155/sci5/3687892",
"ISSN": [
"2090-908X",
"2090-908X"
],
"URL": "http://dx.doi.org/10.1155/sci5/3687892",
"abstract": "<jats:p>Respiratory viral infections, including influenza and coronaviruses, present significant health risks worldwide. The recent COVID‐19 pandemic highlights the urgent need for novel and effective antiviral agents. The host cell protease, transmembrane serine protease 2 (TMPRSS2), facilitates viral pathogenesis by playing a critical role in viral invasion and disease progression. This protease is coexpressed with the viral receptors of angiotensin‐converting enzyme 2 (ACE2) for SARS‐CoV‐2 in the human respiratory tract and plays a significant role in activating viral proteins and spreading. TMPRSS2 activates the coronavirus spike (S) protein and permits membrane fusion and viral entry by cleaving the virus surface glycoproteins. It also activates the hemagglutinin (HA) protein, an enzyme necessary for the spread of influenza virus. TMPRSS2 inhibitors can reduce viral propagation and morbidity by blocking viral entry into respiratory cells and reducing viral spread, inflammation, and disease severity. This review examines the role of TMPRSS2 in viral replication and pathogenicity. It also offers potential avenues to develop targeted antivirals to inhibit TMPRSS2 function, suggesting a possible focus on targeted antiviral development. Ultimately, the review seeks to contribute to improving public health outcomes related to these viral infections.</jats:p>",
"alternative-id": [
"10.1155/sci5/3687892"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-12-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-04-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-04-21"
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-1753-2332",
"affiliation": [],
"authenticated-orcid": false,
"family": "Baby",
"given": "Krishnaprasad",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0009-0001-0186-4906",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vithalkar",
"given": "Megh Pravin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4123-1801",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dastidar",
"given": "Somasish Ghosh",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0402-1143",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mukhopadhyay",
"given": "Chiranjay",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4748-8790",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hamdy",
"given": "Rania",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7691-615X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Soliman",
"given": "Sameh S. M.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0508-1394",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nayak",
"given": "Yogendra",
"sequence": "additional"
}
],
"container-title": "Scientifica",
"container-title-short": "Scientifica",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2025,
4,
21
]
],
"date-time": "2025-04-21T15:18:17Z",
"timestamp": 1745248697000
},
"deposited": {
"date-parts": [
[
2025,
4,
21
]
],
"date-time": "2025-04-21T15:18:18Z",
"timestamp": 1745248698000
},
"editor": [
{
"affiliation": [],
"family": "Rojekar",
"given": "Satish",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/501100001411",
"award": [
"45/21/2022-DDI/BMS"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001411",
"id-type": "DOI"
}
],
"name": "Indian Council of Medical Research"
},
{
"DOI": "10.13039/100019305",
"award": [
"220600104"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100019305",
"id-type": "DOI"
}
],
"name": "Manipal Academy of Higher Education"
},
{
"DOI": "10.13039/100016714",
"award": [
"2401110193"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100016714",
"id-type": "DOI"
}
],
"name": "University of Sharjah"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
22
]
],
"date-time": "2025-04-22T04:03:12Z",
"timestamp": 1745294592256,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2025,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 110,
"start": {
"date-parts": [
[
2025,
4,
21
]
],
"date-time": "2025-04-21T00:00:00Z",
"timestamp": 1745193600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1155/sci5/3687892",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1155",
"published": {
"date-parts": [
[
2025,
1
]
]
},
"published-online": {
"date-parts": [
[
2025,
4,
21
]
]
},
"published-print": {
"date-parts": [
[
2025,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1093/infdis/jiy662",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_1_2"
},
{
"DOI": "10.1183/13993003.02406-2018",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_2"
},
{
"DOI": "10.15585/mmwr.mm6942e3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_2"
},
{
"DOI": "10.1007/s00134-020-05943-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_2"
},
{
"DOI": "10.3390/microorganisms11122974",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_2"
},
{
"DOI": "10.1097/cce.0000000000000107",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_2"
},
{
"DOI": "10.3390/ijms23137314",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_2"
},
{
"DOI": "10.3390/v16050750",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_2"
},
{
"DOI": "10.3390/ijms24098116",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_2"
},
{
"DOI": "10.12688/f1000research.25979.1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_2"
},
{
"DOI": "10.3390/v13061179",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_2"
},
{
"DOI": "10.1128/jvi.01200-18",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_2"
},
{
"DOI": "10.1002/rmv.2225",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_2"
},
{
"DOI": "10.1002/jcp.29785",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_2"
},
{
"DOI": "10.1016/j.jmii.2020.03.034",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_2"
},
{
"DOI": "10.1371/journal.pone.0242763",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_2"
},
{
"DOI": "10.1038/s41598-021-88509-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_2"
},
{
"DOI": "10.1001/jama.2020.22240",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_2"
},
{
"DOI": "10.1111/cts.13001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_2"
},
{
"DOI": "10.1093/ofid/ofaa446",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_2"
},
{
"DOI": "10.1038/s41598-021-91089-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_2"
},
{
"DOI": "10.7326/M20-2496",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_2"
},
{
"DOI": "10.1016/J.CLIM.2020.108652",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_2"
},
{
"DOI": "10.1183/13993003.00858-2020",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_2"
},
{
"DOI": "10.1056/NEJMoa1716197",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_2"
},
{
"DOI": "10.1136/bmj.g2545",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_2"
},
{
"DOI": "10.1007/s40265-018-0981-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_2"
},
{
"DOI": "10.1136/bmj.g2547",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_2"
},
{
"DOI": "10.15585/mmwr.rr7101a1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_2"
},
{
"DOI": "10.1016/j.pharmthera.2020.107512",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_2"
},
{
"DOI": "10.1021/acscentsci.0c00747",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_2"
},
{
"DOI": "10.1016/j.jinf.2022.09.027",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_2"
},
{
"DOI": "10.3390/v16020217",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_2"
},
{
"DOI": "10.1056/NEJMoa2031304",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_2"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_36_2"
},
{
"DOI": "10.1056/NEJMoa2028836",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_2"
},
{
"DOI": "10.1056/NEJMoa2035389",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_2"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_2"
},
{
"DOI": "10.1093/infdis/jit839",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_40_2"
},
{
"DOI": "10.1016/j.jcv.2016.04.019",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_41_2"
},
{
"DOI": "10.7189/JOGH.10.010426",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_42_2"
},
{
"DOI": "10.3390/tropicalmed7110380",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_43_2"
},
{
"DOI": "10.1093/ofid/ofac531",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_44_2"
},
{
"DOI": "10.1002/jmv.24210",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_45_2"
},
{
"DOI": "10.1128/jvi.01815-18",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_46_2"
},
{
"DOI": "10.1128/JVI.00649-19",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_47_2"
},
{
"DOI": "10.1073/pnas.2108728118",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_48_2"
},
{
"DOI": "10.3389/fmicb.2019.01327",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_49_2"
},
{
"DOI": "10.1371/journal.ppat.1003774",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_50_2"
},
{
"DOI": "10.1136/jclinpath-2020-206987",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_51_2"
},
{
"DOI": "10.1006/geno.1997.4845",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_52_2"
},
{
"DOI": "10.2353/ajpath.2010.090665",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_53_2"
},
{
"DOI": "10.1158/2159-8290.CD-13-1010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_54_2"
},
{
"DOI": "10.1371/journal.pone.0035876",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_55_2"
},
{
"DOI": "10.17219/acem/163409",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_56_2"
},
{
"DOI": "10.1038/s41591-020-01227-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_57_2"
},
{
"DOI": "10.1136/gutjnl-2020-320953",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_58_2"
},
{
"DOI": "10.3390/IJMS232314766",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_59_2"
},
{
"DOI": "10.1128/jvi.03372-12",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_60_2"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_61_2"
},
{
"DOI": "10.1128/jvi.01542-10",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_62_2"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_63_2"
},
{
"DOI": "10.15252/embj.2021107821",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_64_2"
},
{
"DOI": "10.1080/07391102.2020.1767690",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_65_2"
},
{
"DOI": "10.3390/genes12010019",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_66_2"
},
{
"DOI": "10.1016/j.cbi.2021.109583",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_67_2"
},
{
"DOI": "10.3389/fphar.2023.1085509",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_68_2"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_69_2"
},
{
"DOI": "10.1128/jvi.02202-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_70_2"
},
{
"DOI": "10.1128/jvi.02232-10",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_71_2"
},
{
"DOI": "10.1016/j.omtn.2021.10.016",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_72_2"
},
{
"DOI": "10.1155/2021/2706789",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_73_2"
},
{
"DOI": "10.1128/jvi.00140-10",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_74_2"
},
{
"DOI": "10.1128/jvi.00128-22",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_75_2"
},
{
"DOI": "10.3390/v15102124",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_76_2"
},
{
"DOI": "10.26508/lsa.202000786",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_77_2"
},
{
"DOI": "10.1016/j.phymed.2020.153396",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_78_2"
},
{
"DOI": "10.3390/ijms232012522",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_79_2"
},
{
"DOI": "10.1016/j.jtcme.2021.04.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_80_2"
},
{
"DOI": "10.1016/j.virusres.2020.198146",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_81_2"
},
{
"DOI": "10.1016/j.celrep.2020.108254",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_82_2"
},
{
"DOI": "10.1016/j.celrep.2020.108016",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_83_2"
},
{
"DOI": "10.1073/pnas.0800502105",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_84_2"
},
{
"DOI": "10.1111/1348-0421.12945",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_85_2"
},
{
"DOI": "10.1074/jbc.RA118.001273",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_86_2"
},
{
"DOI": "10.1093/molbev/msab327",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_87_2"
},
{
"DOI": "10.1186/s12863-023-01169-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_88_2"
},
{
"DOI": "10.1128/jvi.00474-22",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_89_2"
},
{
"DOI": "10.1016/S2666-5247(23)00144-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_90_2"
},
{
"DOI": "10.1038/s41392-020-0184-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_91_2"
},
{
"DOI": "10.1038/s41467-022-31840-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_92_2"
},
{
"DOI": "10.1016/j.bpj.2015.11.2843",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_93_2"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_94_2"
},
{
"DOI": "10.1038/s41586-022-04661-w",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_95_2"
},
{
"DOI": "10.1038/s41467-022-33911-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_96_2"
},
{
"DOI": "10.1016/j.jbc.2022.102643",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_97_2"
},
{
"DOI": "10.1007/s00894-022-05206-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_98_2"
},
{
"DOI": "10.1038/s41589-022-01059-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_99_2"
},
{
"DOI": "10.1038/s41564-021-00908-w",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_100_2"
},
{
"DOI": "10.1128/jvi.01387-16",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_101_2"
},
{
"DOI": "10.1016/j.virol.2023.109889",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_102_2"
},
{
"DOI": "10.3390/v12101174",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_103_2"
},
{
"DOI": "10.3390/v16111798",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_104_2"
},
{
"DOI": "10.1128/jvi.00102-24",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_105_2"
},
{
"DOI": "10.3389/fgeed.2023.1320180",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_106_2"
},
{
"DOI": "10.1371/journal.ppat.1003151",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_107_2"
},
{
"DOI": "10.1099/jgv.0.001274",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_108_2"
},
{
"DOI": "10.1128/jvi.03677-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_109_2"
},
{
"DOI": "10.1128/JVI.00906-21",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_110_2"
},
{
"DOI": "10.1590/1678-4685-gmb-2020-0484",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_111_2"
},
{
"DOI": "10.1093/infdis/jiv246",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_112_2"
},
{
"DOI": "10.1371/journal.pone.0281750",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_113_2"
},
{
"DOI": "10.3389/fgene.2021.667231",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_114_2"
},
{
"DOI": "10.1073/pnas.2021450118",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_115_2"
},
{
"DOI": "10.1038/srep29430",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_116_2"
},
{
"DOI": "10.1074/jbc.RA120.012635",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_117_2"
},
{
"DOI": "10.1128/JVI.02693-15",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_118_2"
},
{
"DOI": "10.1128/JVI.03799-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_119_2"
},
{
"DOI": "10.1042/BJ20130101",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_120_2"
},
{
"DOI": "10.5812/jhgg.119384",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_121_2"
},
{
"DOI": "10.3390/v15020271",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_122_2"
},
{
"DOI": "10.1038/s41598-020-64368-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_123_2"
},
{
"DOI": "10.15252/embj.20105114",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_124_2"
},
{
"DOI": "10.1073/pnas.2002589117",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_125_2"
},
{
"DOI": "10.18632/aging.104015",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_126_2"
},
{
"DOI": "10.1128/JVI.00128-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_127_2"
},
{
"DOI": "10.26508/lsa.202101116",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_128_2"
},
{
"DOI": "10.1128/jvi.01903-23",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_129_2"
},
{
"DOI": "10.1007/s11030-021-10209-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_130_2"
},
{
"DOI": "10.3390/v15071500",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_131_2"
},
{
"DOI": "10.1016/j.vaccine.2012.10.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_132_2"
},
{
"DOI": "10.3389/fcell.2021.717587",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_133_2"
},
{
"DOI": "10.3389/fmolb.2021.666626",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_134_2"
},
{
"DOI": "10.1038/s42003-022-03613-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_135_2"
},
{
"DOI": "10.3389/fphar.2022.870493",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_136_2"
},
{
"DOI": "10.1016/j.ebiom.2021.103255",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_137_2"
},
{
"DOI": "10.3390/biom13091339",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_138_2"
},
{
"DOI": "10.1371/journal.pcbi.1008461",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_139_2"
},
{
"DOI": "10.1007/s40265-013-0150-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_140_2"
},
{
"DOI": "10.1002/ijc.32238",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_141_2"
},
{
"DOI": "10.1124/jpet.117.240317",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_142_2"
},
{
"DOI": "10.1016/j.lfs.2022.121074",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_143_2"
},
{
"DOI": "10.1021/acs.jmedchem.0c00913",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_144_2"
},
{
"DOI": "10.1016/j.stem.2020.11.009",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_145_2"
},
{
"DOI": "10.1073/pnas.2221809120",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_146_2"
},
{
"DOI": "10.1016/j.ejca.2022.08.025",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_147_2"
},
{
"DOI": "10.1038/s41392-022-01084-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_148_2"
},
{
"DOI": "10.1021/acsptsci.0c00221",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_149_2"
},
{
"DOI": "10.1016/j.ejphar.2021.173922",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_150_2"
},
{
"DOI": "10.1080/17460441.2022.2029843",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_151_2"
},
{
"DOI": "10.7454/ijmcb.v1i2.1001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_152_2"
},
{
"DOI": "10.3906/biy-2005-112",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_153_2"
},
{
"DOI": "10.1016/j.imu.2021.100725",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_154_2"
},
{
"DOI": "10.3390/v12111325",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_155_2"
},
{
"DOI": "10.1038/s41598-024-65296-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_156_2"
},
{
"DOI": "10.1016/j.jmgm.2020.107710",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_157_2"
},
{
"DOI": "10.1002/jcb.30528",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_158_2"
},
{
"DOI": "10.1080/07391102.2020.1758791",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_159_2"
},
{
"DOI": "10.1016/j.biochi.2017.07.016",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_160_2"
},
{
"DOI": "10.1016/j.eclinm.2021.100849",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_161_2"
},
{
"DOI": "10.1128/jvi.01294-10",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_162_2"
},
{
"DOI": "10.1021/acs.jpclett.2c00967",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_163_2"
},
{
"DOI": "10.3389/fcimb.2024.1355809",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_164_2"
},
{
"DOI": "10.1158/2159-8290.CD-20-0451",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_165_2"
},
{
"DOI": "10.1016/j.ejps.2020.105495",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_166_2"
},
{
"DOI": "10.1038/s41598-024-67633-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_167_2"
},
{
"DOI": "10.1128/jvi.01427-14",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_168_2"
}
],
"reference-count": 168,
"references-count": 168,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1155/sci5/3687892"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Exploring TMPRSS2 Drug Target to Combat Influenza and Coronavirus Infection",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "2025"
}
