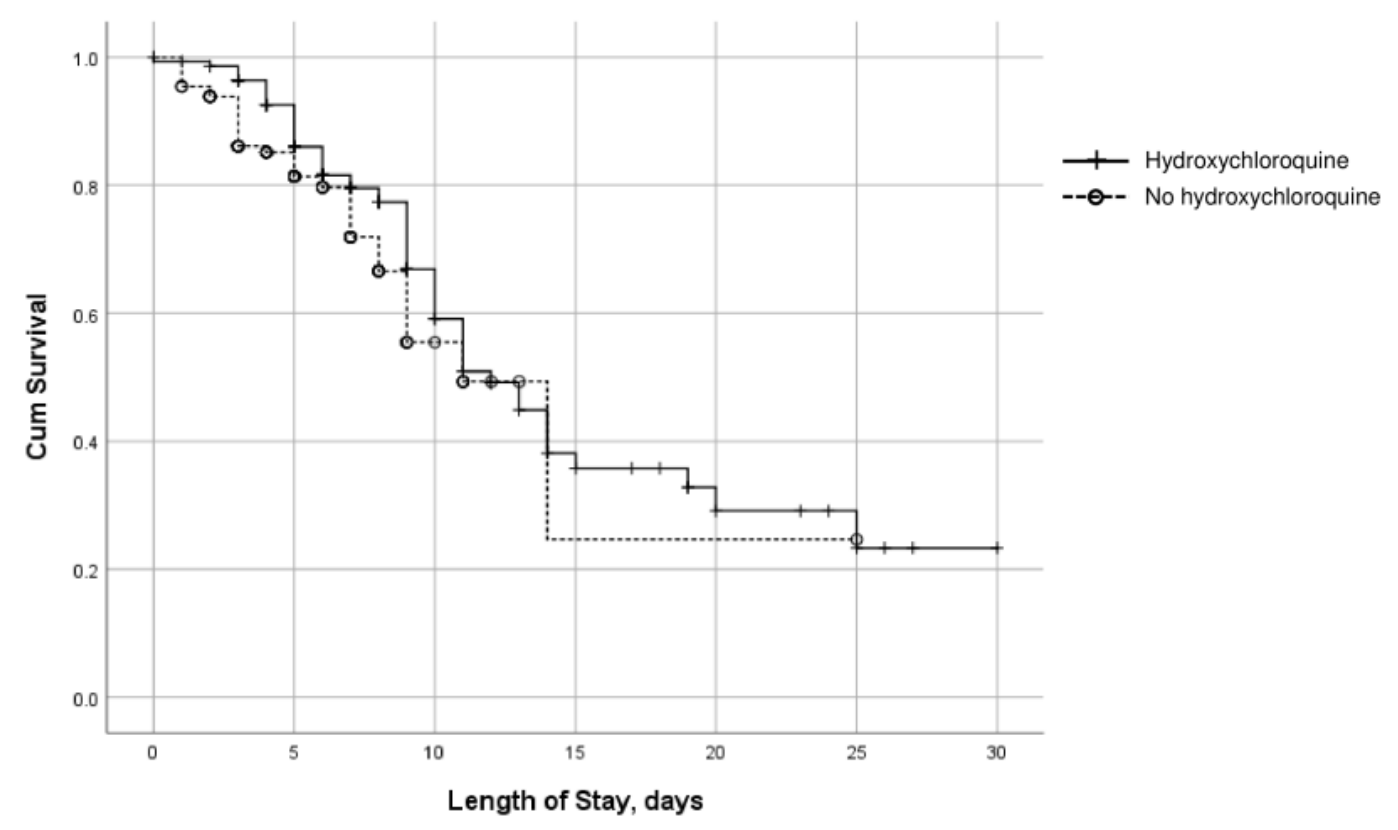
Impact of hydroxychloroquine on disease progression and ICU admissions in patients with SARS-CoV-2 infection
et al., American Journal of Health-System Pharmacy, doi:10.1093/ajhp/zxab056, Feb 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
This paper has inconsistent values - the number of treatment and control patients differs in the text and Table 1, we have used treatment 188 and control 148. Retrospective 336 hospitalized patients in the USA showing higher mortality, ICU admission, and intubation with treatment. Confounding by indication is likely. Time varying confounding is also likely due to declining usage over the early period when overall treatment protocols were also improving dramatically. Authors and reviewers appear to be unfamiliar with either of these.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
This study is excluded in the after exclusion results of meta-analysis:
substantial confounding by time likely due to declining usage over the early stages of the pandemic when overall treatment protocols improved dramatically; substantial unadjusted confounding by indication likely.
|
risk of death, 19.1% higher, RR 1.19, p = 0.60, treatment 56 of 188 (29.8%), control 37 of 148 (25.0%).
|
|
risk of mechanical ventilation, 460.7% higher, RR 5.61, p < 0.001, treatment 64 of 188 (34.0%), control 9 of 148 (6.1%), adjusted per study, odds ratio converted to relative risk.
|
|
risk of ICU admission, 463.4% higher, RR 5.63, p < 0.001, treatment 67 of 188 (35.6%), control 9 of 148 (6.1%), adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Awad et al., 18 Feb 2021, retrospective, USA, peer-reviewed, 4 authors.
Impact of hydroxychloroquine on disease progression and ICU admissions in patients with SARS-CoV-2 infection
American Journal of Health-System Pharmacy, doi:10.1093/ajhp/zxab056
Purpose. To evaluate whether use of hydroxychloroquine was associated with a reduced likelihood of intensive care unit (ICU) admission in patients with coronavirus disease 2019 (COVID-19) in the early weeks of the pandemic. Methods. A retrospective, observational cohort study was conducted to determine selected treatment outcomes in 336 patients hospitalized with COVID-19 at an acute care community hospital in the Hudson Valley region of New York from March 20 to April 20, 2020. Eligibility included admission to the hospital, a laboratory-confirmed diagnosis of SARS-CoV-2 infection, and no need for intubation or intensive care at admission. The median (interquartile range) ages of patients who received hydroxychloroquine (n = 188) and those who did not (n = 148) were 68 (58-82) and 64 (51-73) years, respectively. In a multivariable model that included age, gender, obesity, diabetes, and hydroxychloroquine use, patients who received hydroxychloroquine were significantly more likely than those not treated with the drug to be transferred to an ICU (odds ratio, [OR], 8.1; 95% confidence interval [CI]: 3.8-17) and significantly more likely to be intubated (OR, 7.99; 95% CI, 3.76-16.91); these associations were not influenced by disease severity. In-hospital mortality did not differ significantly with disease severity between those who did and those who did not receive hydroxychloroquine.
Conclusion. Hydroxychloroquine use was significantly associated with increased risks of ICU admission and intubation in patients with mild, moderate, and severe symptoms of COVID-19. There were no significant between-group differences in mortality with use vs nonuse of hydroxychloroquine.
Disclosures The authors have declared no potential conflicts of interest.
REVIEW & RECERTIFICATION REWARD PROGRAM The Right Course for Your Career
Pay $0 for Enrollment and Exam Preparation Resources
References
Achan, Talisuna, Erhart, Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria, Malar J
Ben-Zvi, Kivity, Langevitz, Hydroxychloroquine: from malaria to autoimmunity, Clinic Rev Allerg Immunol
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19
Chen, Hu, Zhang, Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv, doi:10.1101/2020.03.22.20040758
Downes, Chiotos, Fitzgerald, Rational dosing of hydroxychloroquine for COVID-19, OSF Preprints
Geleris, Sun, Platt, Observational study of hydroxychloroquine in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2012410
Giudicessi, Noseworthy, Freidman, Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19)
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China. Published online, N Engl J Med, doi:10.1056/NEJMMoa2002032
Magagnoli, Narendran, Pereira, Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19, doi:10.1101/2020.04.16.20065920
Nika, Blachley, Edwards, Are long-term chloroquine or hydroxychloroquine users being checked regularly for toxic maculopathy?, JAMA Ophthalmol
Perinel, Launay, Botelho-Nevers, Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients, Clin Infect Dis, doi:10.1093/cid/ciaa394
Rosenberg, Dufort, Udo, Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State, JAMA, doi:10.1001/jama.2020.8630
Self, Semler, Leither, Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial, Published, doi:10.1101/2020.04.10.20060558
Vincent, Bergeron, Benjannet, Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol J
Whitley, Ball, Statistics review 4: sample size calculations, Critical Care
Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2. Published online March 9, Clin Infect Dis, doi:10.1093/cid/ciaa237
DOI record:
{
"DOI": "10.1093/ajhp/zxab056",
"ISSN": [
"1079-2082",
"1535-2900"
],
"URL": "http://dx.doi.org/10.1093/ajhp/zxab056",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Purpose</jats:title>\n <jats:p>To evaluate whether use of hydroxychloroquine was associated with a reduced likelihood of intensive care unit (ICU) admission in patients with coronavirus disease 2019 (COVID-19) in the early weeks of the pandemic.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A retrospective, observational cohort study was conducted to determine selected treatment outcomes in 336 patients hospitalized with COVID-19 at an acute care community hospital in the Hudson Valley region of New York from March 20 to April 20, 2020. Eligibility included admission to the hospital, a laboratory-confirmed diagnosis of SARS-CoV-2 infection, and no need for intubation or intensive care at admission. The median (interquartile range) ages of patients who received hydroxychloroquine (n = 188) and those who did not (n = 148) were 68 (58-82) and 64 (51-73) years, respectively. In a multivariable model that included age, gender, obesity, diabetes, and hydroxychloroquine use, patients who received hydroxychloroquine were significantly more likely than those not treated with the drug to be transferred to an ICU (odds ratio, [OR], 8.1; 95% confidence interval [CI]: 3.8-17) and significantly more likely to be intubated (OR, 7.99; 95% CI, 3.76-16.91); these associations were not influenced by disease severity. In-hospital mortality did not differ significantly with disease severity between those who did and those who did not receive hydroxychloroquine.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Hydroxychloroquine use was significantly associated with increased risks of ICU admission and intubation in patients with mild, moderate, and severe symptoms of COVID-19. There were no significant between-group differences in mortality with use vs nonuse of hydroxychloroquine.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Montefiore Nyack Hospital, Nyack, NY, USA"
}
],
"family": "Awad",
"given": "Nirvana",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Montefiore Nyack Hospital, Nyack, NY, USA"
}
],
"family": "Schiller",
"given": "Daryl S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Montefiore Nyack Hospital, Nyack, NY, USA"
}
],
"family": "Fulman",
"given": "Magda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Montefiore Nyack Hospital, Nyack, NY, USA"
}
],
"family": "Chak",
"given": "Azfar",
"sequence": "additional"
}
],
"container-title": "American Journal of Health-System Pharmacy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
18
]
],
"date-time": "2021-02-18T14:26:48Z",
"timestamp": 1613658408000
},
"deposited": {
"date-parts": [
[
2021,
3,
31
]
],
"date-time": "2021-03-31T10:01:40Z",
"timestamp": 1617184900000
},
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T16:30:36Z",
"timestamp": 1711816236673
},
"is-referenced-by-count": 7,
"issue": "8",
"issued": {
"date-parts": [
[
2021,
2,
18
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2021,
2,
18
]
]
},
"published-print": {
"date-parts": [
[
2021,
3,
31
]
]
}
},
"language": "en",
"link": [
{
"URL": "http://academic.oup.com/ajhp/advance-article-pdf/doi/10.1093/ajhp/zxab056/36314759/zxab056.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ajhp/article-pdf/78/8/689/36808218/zxab056.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ajhp/article-pdf/78/8/689/36808218/zxab056.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "689-696",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
2,
18
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
18
]
]
},
"published-other": {
"date-parts": [
[
2021,
4,
15
]
]
},
"published-print": {
"date-parts": [
[
2021,
3,
31
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Johns Hopkins University & Medicine",
"key": "2021033109414455300_CIT0001"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2021033109414455300_CIT0002"
},
{
"DOI": "10.1186/1743-422X-2-69",
"article-title": "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread",
"author": "Vincent",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Virol J.",
"key": "2021033109414455300_CIT0003",
"volume": "2",
"year": "2005"
},
{
"author": "Bhimraj",
"key": "2021033109414455300_CIT0004",
"year": "2020"
},
{
"DOI": "10.1007/s12016-010-8243-x",
"article-title": "Hydroxychloroquine: from malaria to autoimmunity",
"author": "Ben-Zvi",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Clinic Rev Allerg Immunol.",
"key": "2021033109414455300_CIT0005",
"volume": "42",
"year": "2012"
},
{
"DOI": "10.1186/1475-2875-10-144",
"article-title": "Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria",
"author": "Achan",
"doi-asserted-by": "crossref",
"first-page": "144",
"journal-title": "Malar J.",
"key": "2021033109414455300_CIT0006",
"volume": "10",
"year": "2011"
},
{
"DOI": "10.1016/j.mayocp.2020.03.024",
"article-title": "Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19)",
"author": "Giudicessi",
"doi-asserted-by": "crossref",
"journal-title": "Mayo Clin Proc.",
"key": "2021033109414455300_CIT0007",
"year": "2020"
},
{
"DOI": "10.1001/jamaophthalmol.2014.1720",
"article-title": "Are long-term chloroquine or hydroxychloroquine users being checked regularly for toxic maculopathy?",
"author": "Nika",
"doi-asserted-by": "crossref",
"first-page": "1199",
"issue": "10",
"journal-title": "JAMA Ophthalmol",
"key": "2021033109414455300_CIT0008",
"volume": "132",
"year": "2014"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"issue": "15",
"journal-title": "Clin Infect Dis.",
"key": "2021033109414455300_CIT0009",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa394",
"article-title": "Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients",
"author": "Perinel",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "2021033109414455300_CIT0010",
"year": "2020"
},
{
"author": "Downes",
"key": "2021033109414455300_CIT0011",
"year": "2020"
},
{
"DOI": "10.1186/cc1521",
"article-title": "Statistics review 4: sample size calculations",
"author": "Whitley",
"doi-asserted-by": "crossref",
"first-page": "335",
"journal-title": "Critical Care",
"key": "2021033109414455300_CIT0012",
"volume": "6",
"year": "2002"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with COVID-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"issue": "25",
"journal-title": "N Engl J Med.",
"key": "2021033109414455300_CIT0013",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8630",
"article-title": "Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State",
"author": "Rosenberg",
"doi-asserted-by": "crossref",
"journal-title": "JAMA.",
"key": "2021033109414455300_CIT0014",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22240",
"article-title": "Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial",
"author": "Self",
"doi-asserted-by": "crossref",
"journal-title": "JAMA.",
"key": "2021033109414455300_CIT0015",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial",
"author": "Tang",
"journal-title": "medRxiv",
"key": "2021033109414455300_CIT0016",
"year": "2020"
},
{
"article-title": "Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial",
"author": "Chen",
"journal-title": "medRxiv",
"key": "2021033109414455300_CIT0017",
"year": "2020"
},
{
"article-title": "Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19",
"author": "Magagnoli",
"journal-title": "medRxiv",
"key": "2021033109414455300_CIT0018",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med.",
"key": "2021033109414455300_CIT0019",
"year": "2020"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ajhp/article/78/8/689/6144083"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health Policy",
"Pharmacology"
],
"subtitle": [],
"title": "Impact of hydroxychloroquine on disease progression and ICU admissions in patients with SARS-CoV-2 infection",
"type": "journal-article",
"volume": "78"
}
