BHV3500-203: Phase 2/3: Double-Blind, Randomized, Placebo Controlled, Safety and Efficacy Trial of Zavegepant (BHV-3500) Intranasal (IN) for Hospitalized Patients With COVID-19 Requiring Supplemental Oxygen
et al., NCT04346615, BHV-3500, NCT04346615, May 2024
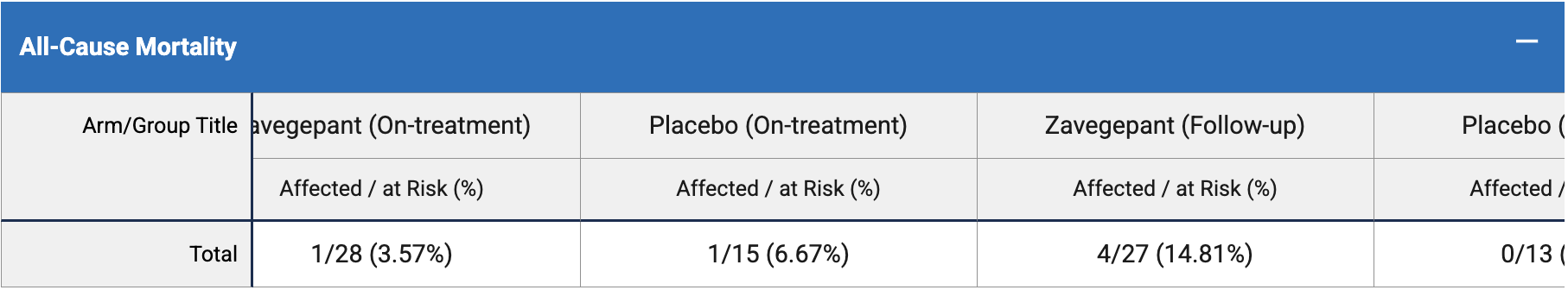
RCT 47 hospitalized COVID-19 patients in the USA showing no significant differences with zavegepant treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 167.9% higher, RR 2.68, p = 0.40, treatment 5 of 28 (17.9%), control 1 of 15 (6.7%).
|
|
risk of ICU admission, 25.0% higher, RR 1.25, p = 1.00, treatment 7 of 28 (25.0%), control 3 of 15 (20.0%).
|
|
risk of no recovery, 11.1% higher, RR 1.11, p = 0.47, treatment mean 5.0 (±1.81) n=20, control mean 4.5 (±1.86) n=11, relative 6-point scale score, day 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Amali et al., 2 May 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04346615 (history) (BHV-3500).
Contact: ClinicalTrials.gov_Inquires@pfizer.com.
Zavegepant is a small molecule calcitonin gene-related peptide (CGRP) receptor antagonist.