Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2020.6319, PATCH, Sep 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
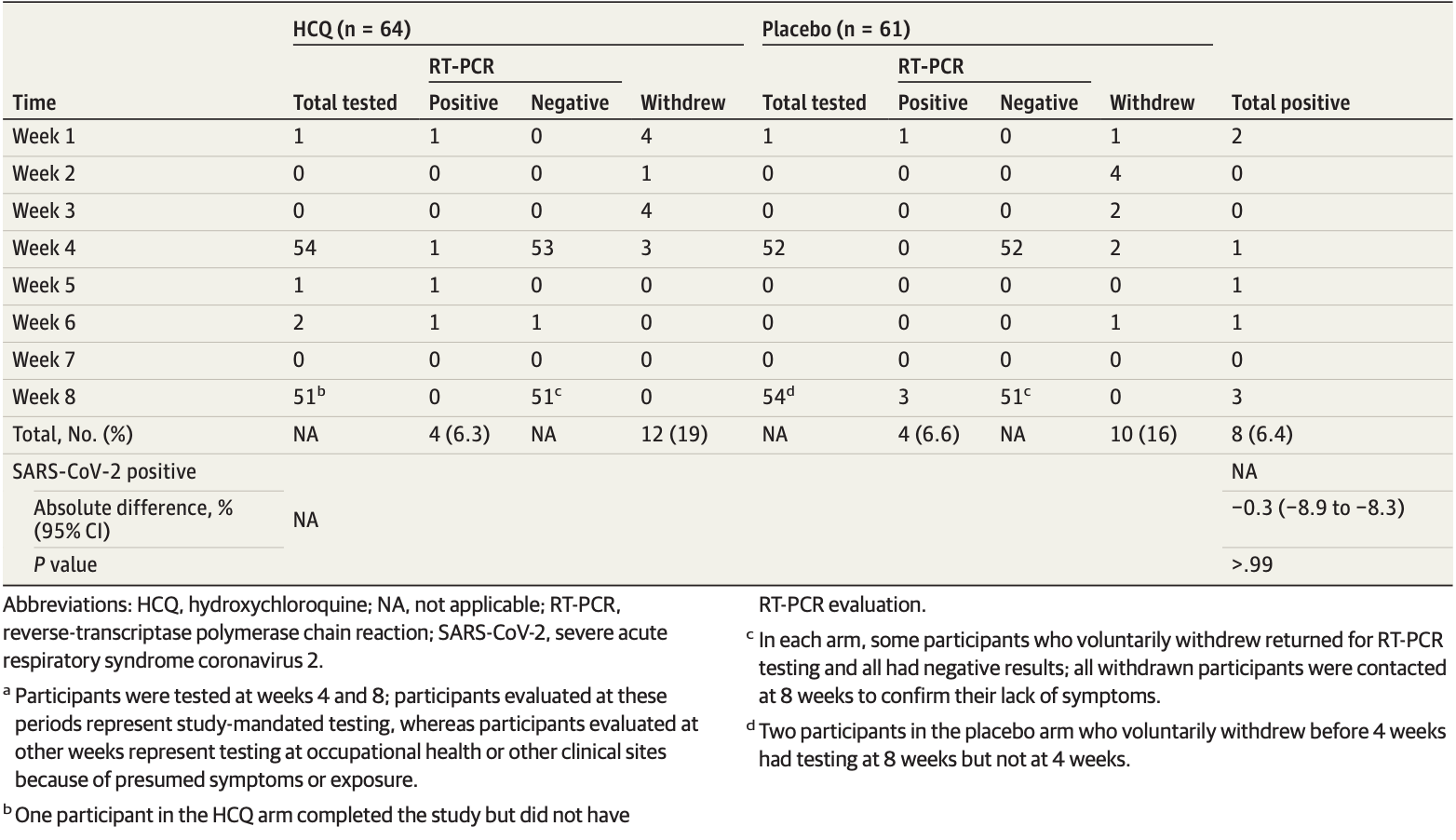
Very small early-terminated underpowered PrEP RCT with 64/61 HCQ/control patients and only 8 infections, HCQ infection rate 6.3% versus control 6.6%, RR 0.95 [0.25 - 3.64].
There was no hospitalization or death, no significant difference in QTc, no severe adverse events, no cardiac events (e.g., syncope and arrhythmias) observed. Medication adherence was 81%. Therapeutic levels of HCQ may not have been reached by the time of the infection in the first week.
2 infections were reported to be after discontinuation of the medication, but the authors do not specify which arm these were in. Hypothetically, if these were both in the HCQ arm, the resulting RR for treatment would be much lower.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of case, 5.0% lower, RR 0.95, p = 1.00, treatment 4 of 64 (6.2%), control 4 of 61 (6.6%), NNT 325.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Abella et al., 30 Sep 2020, Randomized Controlled Trial, USA, peer-reviewed, 18 authors, study period 9 April, 2020 - 14 July, 2020, PATCH trial.
Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers
JAMA Internal Medicine, doi:10.1001/jamainternmed.2020.6319
and the Prevention and Treatment of COVID-19 With Hydroxychloroquine (PATCH) Investigators IMPORTANCE Health care workers (HCWs) caring for patients with coronavirus disease 2019 (COVID-19) are at risk of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, to our knowledge, there is no effective pharmacologic prophylaxis for individuals at risk. OBJECTIVE To evaluate the efficacy of hydroxychloroquine to prevent transmission of SARS-CoV-2 in hospital-based HCWs with exposure to patients with COVID-19 using a pre-exposure prophylaxis strategy. DESIGN, SETTING, AND PARTICIPANTS This randomized, double-blind, placebo-controlled clinical trial (the Prevention and Treatment of COVID-19 With Hydroxychloroquine Study) was conducted at 2 tertiary urban hospitals, with enrollment from April 9, 2020, to July 14, 2020; follow-up ended August 4, 2020. The trial randomized 132 full-time, hospital-based HCWs (physicians, nurses, certified nursing assistants, emergency technicians, and respiratory therapists), of whom 125 were initially asymptomatic and had negative results for SARS-CoV-2 by nasopharyngeal swab. The trial was terminated early for futility before reaching a planned enrollment of 200 participants. INTERVENTIONS Hydroxychloroquine, 600 mg, daily, or size-matched placebo taken orally for 8 weeks. MAIN OUTCOMES AND MEASURES The primary outcome was the incidence of SARS-CoV-2 infection as determined by a nasopharyngeal swab during the 8 weeks of treatment. Secondary outcomes included adverse effects, treatment discontinuation, presence of SARS-CoV-2 antibodies, frequency of QTc prolongation, and clinical outcomes for SARS-CoV-2-positive participants.
RESULTS Of the 132 randomized participants (median age, 33 years [range, 20-66 years]; 91 women [69%]), 125 (94.7%) were evaluable for the primary outcome. There was no significant difference in infection rates in participants randomized to receive hydroxychloroquine compared with placebo (4 of 64 [6.3%] vs 4 of 61 [6.6%]; P > .99). Mild adverse events were more common in participants taking hydroxychloroquine compared with placebo (45% vs 26%; P = .04); rates of treatment discontinuation were similar in both arms (19% vs 16%; P = .81). The median change in QTc (baseline to 4-week evaluation) did not differ between arms (hydroxychloroquine: 4 milliseconds; 95% CI, −9 to 17; vs placebo: 3 milliseconds; 95% CI, −5 to 11; P = .98). Of the 8 participants with positive results for SARS-CoV-2 (6.4%), 6 developed viral symptoms; none required hospitalization, and all clinically recovered.
CONCLUSIONS AND RELEVANCE In this randomized clinical trial, although limited by early termination, there was no clinical benefit of hydroxychloroquine administered daily for 8 weeks as pre-exposure prophylaxis in hospital-based HCWs exposed to patients with COVID-19.
Serological testing for the presence of anti-spike protein RBD IgM and IgG and nucleocapsid protein IgG (eTable 3 in Supplement 3) demonstrated that only 2 participants had anti-nucleocapsid IgG at baseline. Both participants had a negative SARS-CoV-2 RT-P CR test result, and these participants did not possess anti-spike protein RBD IgG at baseline. At the end of the 8 weeks, there were more positive participants treated with hydroxychloroquine (4 [7.4%]) compared with placebo (2 [3.7%]) who had an IgG antibody against SARS-CoV-2 (P = .40). All participants who developed antibodies also converted to SARS-CoV-2 positive status (eTable 4 in Supplement 3). At least 1 dose of study medication was taken by 65 participants in each arm; therefore, these participants were evaluable for adverse events (Table 3 ). The mean (SD) percentage of total pill counts prescribed that were actually taken during study treatment was 97% (8%) (hydroxychloroquine) and 98% (4%) (placebo). No participants in this study experienced grade 3 or 4 adverse events on the Common Toxicity Criteria for Adverse Events scale, hospitalizations, or death. However, there was a significant increase in any adverse events in the hydroxychloroquine arm vs placebo (45% vs 26%; P = .03), with increased diarrhea in participants receiving hydroxychloroquine compared with placebo (32% vs 12%; P = .01). No cardiac events (eg, syncope and arrhythmias) were observed. There was no significant difference in the median of..
References
Bampoe, Lucas, Neall, A cross-sectional study of immune seroconversion to SARS-CoV-2 in frontline maternity health professionals, Anaesthesia, doi:10.1111/anae.15229
Chu, Akl, Duda, Solo, Yaacoub et al., COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis, Lancet, doi:10.1016/S0140-6736(20)31142-9
Giudicessi, Noseworthy, Friedman, Ackerman, Ml et al., Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19), Mayo Clin Proc, doi:10.1016/j.mayocp.2020.05.005
Grau-Pujol, Camprubí, Marti-Soler, Fernández-Pardos, Guinovart et al., Pre-exposure prophylaxis with hydroxychloroquine for high-risk healthcare workers during the COVID-19 pandemic: a structured summary of a study protocol for a multicentre, double-blind randomized controlled trial, Trials, doi:10.1186/s13063-020-04621-7
Hernandez, Roman, Pasupuleti, Barboza, White, Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review, Ann Intern Med, doi:10.7326/M20-2496
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19-preliminary Report, N Engl J Med, doi:10.1056/NEJMoa2021436
Maisonnasse, Guedj, Contreras, Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates, Nature, doi:10.1038/s41586-020-2558-4
Nanni, Viale, Vertogen, PROTECT Trial: a cluster-randomized study with hydroxychloroquine versus observational support for prevention or early-phase treatment of coronavirus disease (COVID-19): a structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04527-4
Olender, Perez, Go, Remdesivir for severe COVID-19 versus a cohort receiving standard of care, Clin Infect Dis, doi:10.1093/cid/ciaa1041
Shippey, Wagler, Collamer, Juurlink, Boulware et al., Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection, Cleve Clin J Med, doi:10.1056/NEJMe2020388
Tai, Shah, Doubeni, Disproportionate impact of COVID-19 on racial and ethnic minorities in the United States, Clin Infect Dis. Published online, doi:10.1093/cid/ciaa815
Weinberger, Chen, Cohen, Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to, JAMA Intern Med, doi:10.1001/jamainternmed.2020.3391?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2020.6319
Wright, Tan, Walmsley, Protecting frontline health care workers from COVID-19 with hydroxychloroquine pre-exposure prophylaxis: a structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada, Trials, doi:10.1186/s13063-020-04577-8
Ye, Zhang, Zhang, Impact of comorbidities on patients with COVID-19: a large retrospective study in Zhejiang, China, J Med Virol. Published, doi:10.1002/jmv.26183
Zhang, Schwartz, Spatial disparities in coronavirus incidence and mortality in the United States: An ecological analysis as of May
DOI record:
{
"DOI": "10.1001/jamainternmed.2020.6319",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2020.6319",
"author": [
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Abella",
"given": "Benjamin S.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Jolkovsky",
"given": "Eliana L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Biney",
"given": "Barbara T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Uspal",
"given": "Julie E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Department of Medicine University of Pennsylvania, Philadelphia"
}
],
"family": "Hyman",
"given": "Matthew C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine University of Pennsylvania, Philadelphia"
}
],
"family": "Frank",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, University of Pennsylvania, Philadelphia"
}
],
"family": "Hensley",
"given": "Scott E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Gill",
"given": "Saar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Vogl",
"given": "Dan T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Maillard",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Babushok",
"given": "Daria V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Huang",
"given": "Alexander C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Nasta",
"given": "Sunita D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Walsh",
"given": "Jennifer C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, Philadelphia"
}
],
"family": "Wiletyo",
"given": "E. Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, Philadelphia"
}
],
"family": "Gimotty",
"given": "Phyllis A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, University of Pennsylvania, Philadelphia"
}
],
"family": "Milone",
"given": "Michael C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Amaravadi",
"given": "Ravi K.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Prevention and Treatment of COVID-19 With Hydroxychloroquine (PATCH) Investigators",
"sequence": "additional"
}
],
"container-title": "JAMA Internal Medicine",
"container-title-short": "JAMA Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
30
]
],
"date-time": "2020-09-30T21:02:12Z",
"timestamp": 1601499732000
},
"deposited": {
"date-parts": [
[
2021,
2,
2
]
],
"date-time": "2021-02-02T05:41:52Z",
"timestamp": 1612244512000
},
"indexed": {
"date-parts": [
[
2024,
4,
5
]
],
"date-time": "2024-04-05T02:15:41Z",
"timestamp": 1712283341847
},
"is-referenced-by-count": 141,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
1
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
2,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2771265/jamainternal_abella_2020_oi_200089_1611344111.08576.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "195",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
2,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
2,
1
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"article-title": "Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020.",
"author": "Weinberger",
"journal-title": "JAMA Intern Med",
"key": "ioi200089r2",
"volume": "e203391",
"year": "2020"
},
{
"DOI": "10.1111/jrh.v36.3",
"article-title": "Spatial disparities in coronavirus incidence and mortality in the United States: An ecological analysis as of May 2020.",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "433",
"issue": "3",
"journal-title": "J Rural Health",
"key": "ioi200089r3",
"volume": "36",
"year": "2020"
},
{
"article-title": "Disproportionate impact of COVID-19 on racial and ethnic minorities in the United States.",
"author": "Tai",
"journal-title": "Clin Infect Dis",
"key": "ioi200089r4"
},
{
"article-title": "Impact of comorbidities on patients with COVID-19: a large retrospective study in Zhejiang, China.",
"author": "Ye",
"journal-title": "J Med Virol",
"key": "ioi200089r5"
},
{
"DOI": "10.1111/anae.15229",
"article-title": "A cross-sectional study of immune seroconversion to SARS-CoV-2 in frontline maternity health professionals.",
"author": "Bampoe",
"doi-asserted-by": "crossref",
"journal-title": "Anaesthesia",
"key": "ioi200089r6",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31142-9",
"article-title": "Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis.",
"author": "Chu",
"doi-asserted-by": "publisher",
"first-page": "1973",
"issue": "10242",
"journal-title": "Lancet",
"key": "ioi200089r7",
"volume": "395",
"year": "2020"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19—preliminary Report.",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "ioi200089r8",
"year": "2020"
},
{
"article-title": "Remdesivir for severe COVID-19 versus a cohort receiving standard of care.",
"author": "Olender",
"journal-title": "Clin Infect Dis",
"key": "ioi200089r9",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04621-7",
"article-title": "Pre-exposure prophylaxis with hydroxychloroquine for high-risk healthcare workers during the COVID-19 pandemic: a structured summary of a study protocol for a multicentre, double-blind randomized controlled trial.",
"author": "Grau-Pujol",
"doi-asserted-by": "publisher",
"first-page": "688",
"issue": "1",
"journal-title": "Trials",
"key": "ioi200089r10",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.7326/M20-2496",
"article-title": "Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review.",
"author": "Hernandez",
"doi-asserted-by": "publisher",
"first-page": "287",
"issue": "4",
"journal-title": "Ann Intern Med",
"key": "ioi200089r11",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.3949/ccjm.85a.17034",
"article-title": "Hydroxychloroquine: an old drug with new relevance.",
"author": "Shippey",
"doi-asserted-by": "publisher",
"first-page": "459",
"issue": "6",
"journal-title": "Cleve Clin J Med",
"key": "ioi200089r12",
"volume": "85",
"year": "2018"
},
{
"DOI": "10.1503/cmaj.200528",
"article-title": "Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection.",
"author": "Juurlink",
"doi-asserted-by": "publisher",
"first-page": "E450",
"issue": "17",
"journal-title": "CMAJ",
"key": "ioi200089r13",
"volume": "192",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2016638",
"article-title": "A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19.",
"author": "Boulware",
"doi-asserted-by": "publisher",
"first-page": "517",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "ioi200089r14",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2020388",
"article-title": "Hydroxychloroquine for the prevention of Covid-19—searching for evidence.",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "585",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "ioi200089r15",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.03.024",
"article-title": "Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19).",
"author": "Giudicessi",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "6",
"journal-title": "Mayo Clin Proc",
"key": "ioi200089r16",
"volume": "95",
"year": "2020"
},
{
"article-title": "Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19).",
"author": "Puntmann",
"journal-title": "JAMA Cardiol",
"key": "ioi200089r18",
"volume": "e203557",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.05.005",
"article-title": "Acute QT interval modifications during hydroxychloroquine-azithromycin treatment in the context of COVID-19 infection.",
"author": "Voisin",
"doi-asserted-by": "publisher",
"first-page": "1696",
"issue": "8",
"journal-title": "Mayo Clin Proc",
"key": "ioi200089r19",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2558-4",
"article-title": "Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates.",
"author": "Maisonnasse",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "ioi200089r21",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04577-8",
"article-title": "Protecting frontline health care workers from COVID-19 with hydroxychloroquine pre-exposure prophylaxis: a structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada.",
"author": "Wright",
"doi-asserted-by": "publisher",
"first-page": "647",
"issue": "1",
"journal-title": "Trials",
"key": "ioi200089r22",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04527-4",
"article-title": "PROTECT Trial: a cluster-randomized study with hydroxychloroquine versus observational support for prevention or early-phase treatment of coronavirus disease (COVID-19): a structured summary of a study protocol for a randomized controlled trial.",
"author": "Nanni",
"doi-asserted-by": "publisher",
"first-page": "689",
"issue": "1",
"journal-title": "Trials",
"key": "ioi200089r23",
"volume": "21",
"year": "2020"
},
{
"key": "ioi200089r1",
"unstructured": "US Centers for Disease Control and Prevention. CDC COVID data tracker. Accessed September 23, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html."
},
{
"key": "ioi200089r17",
"unstructured": "National Cancer Institute. Common terminology criteria for adverse events (CTCAE). Accessed August 16, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm"
},
{
"key": "ioi200089r20",
"unstructured": "Google. Publicly available COVID-19 datasets. Accessed August 16, 2020. https://cloud.google.com/blog/products/data-analytics/publicly-available-covid-19-data-for-analytics"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2771265"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Internal Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers",
"type": "journal-article",
"volume": "181"
}
