Efficacy of Lactococcus lactis Strain Plasma in Patients with Mild COVID-19: A Multicenter, Double-Blinded, Randomized-Controlled Trial (PLATEAU Study)
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-025-01246-8, PLATEAU, jRCTs071210097, Oct 2025
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
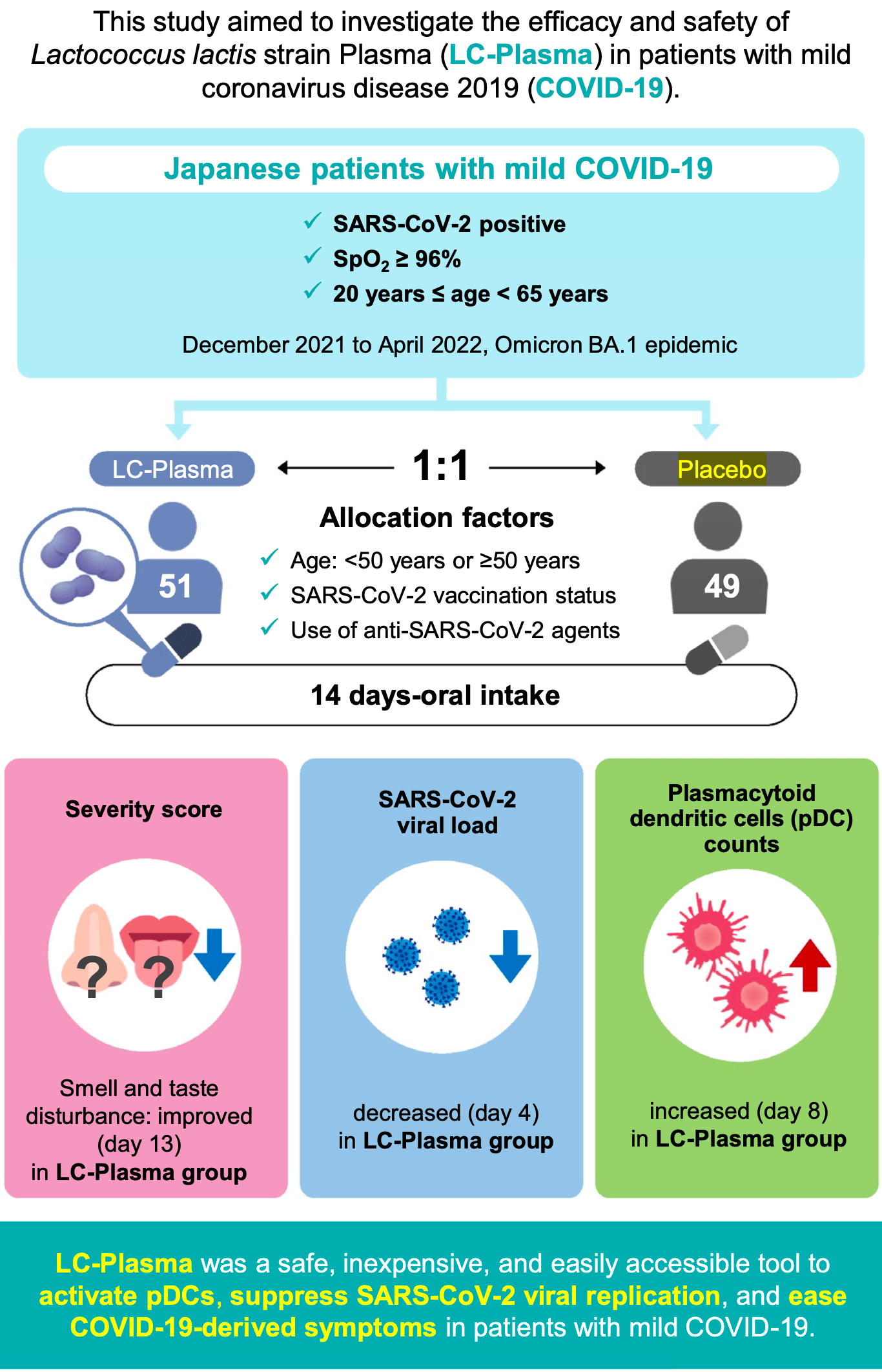
RCT 100 outpatients with mild COVID-19 showing no significant difference in overall symptom severity with Lactococcus lactis strain Plasma (LC-Plasma). However, post hoc analysis revealed a significantly higher proportion of patients without smell and taste disturbances in the LC-Plasma group at later time points (97.8% vs 82.6% on day 13, p=0.030). LC-Plasma showed earlier viral load reduction by day 4 and increased plasmacytoid dendritic cell counts. Authors hypothesize LC-Plasma may suppress neurological damage through pDC activation, facilitating immune response against upper airway symptoms. There were no serious adverse events.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of no recovery, 26.0% lower, RR 0.74, p < 0.001, treatment mean 0.76 (±0.18) n=45, control mean 1.02 (±0.3) n=46, relative total severity score, day 14.
|
|
risk of no viral clearance, 61.6% lower, RR 0.38, p = 0.21, treatment mean 27.9 (±29.3) n=45, control mean 10.7 (±87.5) n=46, relative decrease in viral load, mid-recovery, day 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yamamoto et al., 25 Oct 2025, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 29 authors, study period December 2021 - April 2022, trial jRCTs071210097 (PLATEAU).
Contact: kazukomd@cs.u-ryukyu.ac.jp.
Efficacy of Lactococcus lactis Strain Plasma in Patients with Mild COVID-19: A Multicenter, Double-Blinded, Randomized-Controlled Trial (PLATEAU Study)
Infectious Diseases and Therapy, doi:10.1007/s40121-025-01246-8
Introduction: Coronavirus disease 2019 , caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is still an ongoing public health threat. COVID-19 can be accompanied by prolonged symptoms, known as "long COVID", however, no pharmaceutical treatments are currently available for these symptoms. Lactococcus lactis strain Plasma (LC-Plasma; Lactococcus lactis subsp. lactis JCM 5805) directly activates human plasmacytoid dendritic cells (pDCs) and triggers antiviral immune responses. We hypothesized that LC-Plasma reduced SARS-CoV-2 viral load and eased symptoms in patients with mild COVID-19. Methods: This PLATEAU study enrolled 100 patients with mild COVID-19 during Omicron
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahmad, Cisewski, Xu, Anderson, COVID-19 mortality update-United States, 2022, MMWR Morb Mortal Wkly Rep
Fujii, Jounai, Horie, Effects of heatkilled Lactococcus lactis subsp. lactis JCM 5805 on mucosal and systemic immune parameters, and antiviral reactions to influenza virus in healthy adults; a randomized controlled double-blind study, J Funct Foods
Fujiwata, lactic acid bacterium that activates plasmacytoid dendritic cells, Exp Med
Ishii, Jounai, Tsuji, Plasmacytoid dendritic cells stimulated with Lactococcus lactis strain plasma produce soluble factors to suppress SARS-CoV-2 replication, Biochem Biophys Res Commun
Janowitz, Gablenz, Pattinson, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut
Jounai, Ikado, Sugimura, Ano, Braun et al., Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells, PLoS ONE
Jounai, Sugimura, Ohshio, Fujiwara, Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection, PLoS ONE
Kanauchi, Low, Jounai, Tsuji, Abubakar, Overview of anti-viral effects of probiotics via immune cells in pre-, mid-and post-SARS-CoV2 era, Front Immunol
Khor, Tsuji, Lee, Lactococcus lactis strain plasma intake suppresses the incidence of Dengue fever-like symptoms in healthy Malaysians: a randomized, double-blind, placebo-controlled trial, Nutrients
Lin, Rajagopalan, Gamage, Longitudinal single cell atlas identifies complex temporal relationship between type I interferon response and COVID-19 severity, Nat Commun
Mostert, Hoogland, Huibers, Kaspers, Excess mortality across countries in the Western World since the COVID-19 pandemic: 'our world in data' estimates of January 2020 to, BMJ Public Health
Mukae, Yotsuyanagi, Ohmagari, A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3c-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob Agents Chemother
O'mahoney, Routen, Gillies, The risk of long Covid symptoms: a systematic review and meta-analysis of controlled studies, Nat Commun
Sakata, Sasaki, Jounai, Fujii, Fujiwara, Preventive effect of Lactococcus lactis subsp. lactis JCM 5805 yogurt intake on influenza infection among choolchildren, Health
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs, Clin Infect Dis
Shah, Firmal, Alam, Ganguly, Chattopadhyay, Overview of immune response during SARS-CoV-2 infection: lessons from the past, Front Immunol
Sharetts, Moein, Khan, Doty, Long-term taste and smell outcomes after COVID-19, JAMA Netw Open
Shibata, Kanayama, Haida, Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza Infect Dis Ther in healthy adults in a randomized controlled trial, J Funct Foods
Song, Hui, Hull, Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses, Lancet Respir Med
Sugimura, Jounai, Ohshio, Tanaka, Suwa et al., Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells, Clin Immunol
Sugimura, Takahashi, Jounai, Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus, Br J Nutr
Suzuki, Jounai, Ohshio, Fujii, Fujiwara, Administration of plasmacytoid dendritic cellstimulative lactic acid bacteria enhances antigenspecific immune responses, Biochem Biophys Res Commun
Suzuki, Tsuji, Sugamata, Yamamoto, Yamamoto et al., Administration of plasmacytoid dendritic cell-stimulative lactic acid bacteria is effective against dengue virus infection in mice, Int J Mol Med
Tanaka, Suzuki, Kanayama, The safety evaluation of long-term or excessive intake of the beverage containing Lactococcus lactis subsp. lactis JCM 5805 and resistant maltodextrin-a randomized, double-blind, placebo-controlled, parallelgroup trial, Jpn Pharmacol Ther
Tanaka, Yamamoto, Morimoto, Evaluation of a triage checklist for mild COVID-19 outpatients in predicting subsequent emergency department visits and hospitalization during the isolation period: a single-center retrospective study, J Clin Med
Venet, Ribeiro, Décembre, Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2, Nat Commun
Yamamoto, Hosogaya, Inoue, Efficacy of Lactococcus lactis strain plasma (LC-Plasma) in easing symptoms in patients with mild COVID-19: protocol for an exploratory, multicentre, doubleblinded, randomised controlled trial (PLATEAU study), BMJ Open
DOI record:
{
"DOI": "10.1007/s40121-025-01246-8",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-025-01246-8",
"alternative-id": [
"1246"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "17 August 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2 October 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "25 October 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Kazuko Yamamoto received research funds from Kirin Holdings Co., Ltd. for this study and another research grant from Fisher & Paykel Healthcare Ltd. Tsuyoshi Inoue received research funds from Kirin Holdings Co., Ltd. for this study. It belonged to a donated course from Kyowa Kirin Co., Ltd. Kenta Jounai, Ryohei Tsuji, and Daisuke Fujiwara are employees of Kirin Holdings Co. Ltd. Koichi Izumikawa received a research grant for this study from Kirin Holdings Co., Ltd. Hiroshi Mukae received a research grant from Taisho Pharmaceutical Co. Ltd. All funding agencies, except Kirin Holdings Co., Ltd., played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. Takaya Ikeda, Toyomitsu Sawai, Yosuke Nagayoshi, Koji Hashiguchi, Yoji Futsuki, Yuichi Matsubara, Yosuke Harada, Nobuyuki Ashizawa, Susumu Fukahori, Naoki Iwanaga, Takahiro Takazono, Takashi Kido, Hiroshi Ishimoto, Naoki Hosogaya, Noriho Sakamoto, Masato Tashiro, Takeshi Tanaka, Chizu Fukushima, Kenji Ota, Kosuke Kosai, Akitsugu Furumoto, and Katsunori Yanagihara declare no conflicts of interest."
},
{
"group": {
"label": "Ethical Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The study protocols were inspected and approved (approval no. CRB21-009) in November 2021 by the Clinical Research Review Board of Nagasaki University. This study was registered with the Japan Registry of Clinical Trials (jRCT) (registration number: jRCTs071210097) in December 2021. The trial protocol has been described previously [\n \n ]. Patient enrollment was conducted from December 2021 to April 2022. The study was conducted in accordance with the Declaration of Helsinki, Clinical Trials Act, and other current legal regulations in Japan. Written informed consent was obtained from all enrolled patients who met the eligibility criteria before the intervention. To avoid bias and ensure quality, a third-party entity (Soiken, Inc., Osaka, Japan) performed data collection, management, monitoring, audits, and statistical analyses."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-1357-1676",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yamamoto",
"given": "Kazuko",
"sequence": "first"
},
{
"affiliation": [],
"family": "Inoue",
"given": "Tsuyoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ikeda",
"given": "Takaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sawai",
"given": "Toyomitsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nagayoshi",
"given": "Yosuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hashiguchi",
"given": "Koji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Futsuki",
"given": "Yoji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsubara",
"given": "Yuichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harada",
"given": "Yosuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ashizawa",
"given": "Nobuyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukahori",
"given": "Susumu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iwanaga",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takazono",
"given": "Takahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kido",
"given": "Takashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ishimoto",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hosogaya",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sakamoto",
"given": "Noriho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tashiro",
"given": "Masato",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tanaka",
"given": "Takeshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukushima",
"given": "Chizu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jounai",
"given": "Kenta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsuji",
"given": "Ryohei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fujiwara",
"given": "Daisuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ota",
"given": "Kenji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kosai",
"given": "Kosuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Furumoto",
"given": "Akitsugu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yanagihara",
"given": "Katsunori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Izumikawa",
"given": "Koichi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mukae",
"given": "Hiroshi",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
25
]
],
"date-time": "2025-10-25T07:32:55Z",
"timestamp": 1761377575000
},
"deposited": {
"date-parts": [
[
2025,
10,
25
]
],
"date-time": "2025-10-25T07:32:58Z",
"timestamp": 1761377578000
},
"funder": [
{
"award": [
"H21003539"
],
"name": "Kirin Holdings Co., Ltd."
}
],
"indexed": {
"date-parts": [
[
2025,
10,
25
]
],
"date-time": "2025-10-25T07:37:54Z",
"timestamp": 1761377874809,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
10,
25
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
25
]
],
"date-time": "2025-10-25T00:00:00Z",
"timestamp": 1761350400000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
25
]
],
"date-time": "2025-10-25T00:00:00Z",
"timestamp": 1761350400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-025-01246-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-025-01246-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-025-01246-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2025,
10,
25
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
25
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.15585/mmwr.mm7218a4",
"author": "FB Ahmad",
"doi-asserted-by": "publisher",
"first-page": "493",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "1246_CR1",
"unstructured": "Ahmad FB, Cisewski JA, Xu J, Anderson RN. COVID-19 mortality update—United States, 2022. MMWR Morb Mortal Wkly Rep. 2023;72:493–6.",
"volume": "72",
"year": "2023"
},
{
"key": "1246_CR2",
"unstructured": "Ministry of Health, Labour and Welfare. Visualizing the data: information on COVID-19 infections. https://covid19.mhlw.go.jp/en/. Accessed 2 Oct 2025."
},
{
"DOI": "10.1136/bmjph-2023-000282",
"author": "S Mostert",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Public Health",
"key": "1246_CR3",
"unstructured": "Mostert S, Hoogland M, Huibers M, Kaspers G. Excess mortality across countries in the Western World since the COVID-19 pandemic: ‘our world in data’ estimates of January 2020 to December 2022. BMJ Public Health. 2024;2:e000282.",
"volume": "2",
"year": "2024"
},
{
"DOI": "10.1038/s41467-025-59012-w",
"author": "LL O'Mahoney",
"doi-asserted-by": "publisher",
"first-page": "4249",
"journal-title": "Nat Commun",
"key": "1246_CR4",
"unstructured": "O’Mahoney LL, Routen A, Gillies C, et al. The risk of long Covid symptoms: a systematic review and meta-analysis of controlled studies. Nat Commun. 2025;16:4249.",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1001/jamanetworkopen.2024.7818",
"author": "R Sharetts",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "1246_CR5",
"unstructured": "Sharetts R, Moein ST, Khan R, Doty RL. Long-term taste and smell outcomes after COVID-19. JAMA Netw Open. 2024;7:e247818.",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1371/journal.pone.0032588",
"author": "K Jounai",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "1246_CR6",
"unstructured": "Jounai K, Ikado K, Sugimura T, Ano Y, Braun J, Fujiwara D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE. 2012;7:e32588.",
"volume": "7",
"year": "2012"
},
{
"author": "D Fujiwata",
"first-page": "191",
"journal-title": "Exp Med",
"key": "1246_CR7",
"unstructured": "Fujiwata D. lactic acid bacterium that activates plasmacytoid dendritic cells. Exp Med. 2017;35:191–6.",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.3389/fimmu.2023.1280680",
"author": "O Kanauchi",
"doi-asserted-by": "publisher",
"first-page": "1280680",
"journal-title": "Front Immunol",
"key": "1246_CR8",
"unstructured": "Kanauchi O, Low ZX, Jounai K, Tsuji R, AbuBakar S. Overview of anti-viral effects of probiotics via immune cells in pre-, mid- and post-SARS-CoV2 era. Front Immunol. 2023;14:1280680.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0119055",
"author": "K Jounai",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "1246_CR9",
"unstructured": "Jounai K, Sugimura T, Ohshio K, Fujiwara D. Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLoS ONE. 2015;10:e0119055.",
"volume": "10",
"year": "2015"
},
{
"author": "H Suzuki",
"first-page": "426",
"journal-title": "Int J Mol Med",
"key": "1246_CR10",
"unstructured": "Suzuki H, Tsuji R, Sugamata M, Yamamoto N, Yamamoto N, Kanauchi O. Administration of plasmacytoid dendritic cell-stimulative lactic acid bacteria is effective against dengue virus infection in mice. Int J Mol Med. 2019;43:426–34.",
"volume": "43",
"year": "2019"
},
{
"DOI": "10.1016/j.clim.2013.10.007",
"author": "T Sugimura",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "Clin Immunol",
"key": "1246_CR11",
"unstructured": "Sugimura T, Jounai K, Ohshio K, Tanaka T, Suwa M, Fujiwara D. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin Immunol. 2013;149:509–18.",
"volume": "149",
"year": "2013"
},
{
"DOI": "10.1016/j.jff.2016.03.035",
"author": "T Shibata",
"doi-asserted-by": "publisher",
"first-page": "492",
"journal-title": "J Funct Foods",
"key": "1246_CR12",
"unstructured": "Shibata T, Kanayama M, Haida M, et al. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J Funct Foods. 2016;24:492–500.",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.1016/j.jff.2017.06.011",
"author": "T Fujii",
"doi-asserted-by": "publisher",
"first-page": "513",
"journal-title": "J Funct Foods",
"key": "1246_CR13",
"unstructured": "Fujii T, Jounai K, Horie A, et al. Effects of heat-killed Lactococcus lactis subsp. lactis JCM 5805 on mucosal and systemic immune parameters, and antiviral reactions to influenza virus in healthy adults; a randomized controlled double-blind study. J Funct Foods. 2017;35:513–21.",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.4236/health.2017.94054",
"author": "K Sakata",
"doi-asserted-by": "publisher",
"first-page": "756",
"journal-title": "Health",
"key": "1246_CR14",
"unstructured": "Sakata K, Sasaki Y, Jounai K, Fujii T, Fujiwara D. Preventive effect of Lactococcus lactis subsp. lactis JCM 5805 yogurt intake on influenza infection among choolchildren. Health. 2017;9:756–62.",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.3390/nu13124507",
"author": "CS Khor",
"doi-asserted-by": "publisher",
"journal-title": "Nutrients",
"key": "1246_CR15",
"unstructured": "Khor CS, Tsuji R, Lee HY, et al. Lactococcus lactis strain plasma intake suppresses the incidence of Dengue fever-like symptoms in healthy Malaysians: a randomized, double-blind, placebo-controlled trial. Nutrients. 2021;13:4507.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1017/S0007114515002408",
"author": "T Sugimura",
"doi-asserted-by": "publisher",
"first-page": "727",
"journal-title": "Br J Nutr",
"key": "1246_CR16",
"unstructured": "Sugimura T, Takahashi H, Jounai K, et al. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br J Nutr. 2015;114:727–33.",
"volume": "114",
"year": "2015"
},
{
"DOI": "10.1016/j.bbrc.2018.07.042",
"author": "H Suzuki",
"doi-asserted-by": "publisher",
"first-page": "1315",
"journal-title": "Biochem Biophys Res Commun",
"key": "1246_CR17",
"unstructured": "Suzuki H, Jounai K, Ohshio K, Fujii T, Fujiwara D. Administration of plasmacytoid dendritic cell-stimulative lactic acid bacteria enhances antigen-specific immune responses. Biochem Biophys Res Commun. 2018;503:1315–21.",
"volume": "503",
"year": "2018"
},
{
"DOI": "10.3389/fimmu.2020.01949",
"author": "VK Shah",
"doi-asserted-by": "publisher",
"first-page": "1949",
"journal-title": "Front Immunol",
"key": "1246_CR18",
"unstructured": "Shah VK, Firmal P, Alam A, Ganguly D, Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front Immunol. 2020;11:1949.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2023.04.046",
"author": "H Ishii",
"doi-asserted-by": "publisher",
"first-page": "26",
"journal-title": "Biochem Biophys Res Commun",
"key": "1246_CR19",
"unstructured": "Ishii H, Jounai K, Tsuji R, et al. Plasmacytoid dendritic cells stimulated with Lactococcus lactis strain plasma produce soluble factors to suppress SARS-CoV-2 replication. Biochem Biophys Res Commun. 2023;662:26–30.",
"volume": "662",
"year": "2023"
},
{
"DOI": "10.1136/bmjopen-2022-061172",
"author": "K Yamamoto",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Open",
"key": "1246_CR20",
"unstructured": "Yamamoto K, Hosogaya N, Inoue T, et al. Efficacy of Lactococcus lactis strain plasma (LC-Plasma) in easing symptoms in patients with mild COVID-19: protocol for an exploratory, multicentre, double-blinded, randomised controlled trial (PLATEAU study). BMJ Open. 2022;12:e061172.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"author": "T Janowitz",
"doi-asserted-by": "publisher",
"first-page": "1592",
"journal-title": "Gut",
"key": "1246_CR21",
"unstructured": "Janowitz T, Gablenz E, Pattinson D, et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592–7.",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.3390/jcm11185444",
"author": "Y Tanaka",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Med",
"key": "1246_CR22",
"unstructured": "Tanaka Y, Yamamoto K, Morimoto S, et al. Evaluation of a triage checklist for mild COVID-19 outpatients in predicting subsequent emergency department visits and hospitalization during the isolation period: a single-center retrospective study. J Clin Med. 2022;11:5444.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-36140-9",
"author": "M Venet",
"doi-asserted-by": "publisher",
"first-page": "694",
"journal-title": "Nat Commun",
"key": "1246_CR23",
"unstructured": "Venet M, Ribeiro MS, Décembre E, et al. Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nat Commun. 2023;14:694.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/S2213-2600(21)00125-9",
"author": "WJ Song",
"doi-asserted-by": "publisher",
"first-page": "533",
"journal-title": "Lancet Respir Med",
"key": "1246_CR24",
"unstructured": "Song WJ, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–44.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41467-023-44524-0",
"author": "QXX Lin",
"doi-asserted-by": "publisher",
"first-page": "567",
"journal-title": "Nat Commun",
"key": "1246_CR25",
"unstructured": "Lin QXX, Rajagopalan D, Gamage AM, et al. Longitudinal single cell atlas identifies complex temporal relationship between type I interferon response and COVID-19 severity. Nat Commun. 2024;15:567.",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciac180",
"author": "LD Saravolatz",
"doi-asserted-by": "publisher",
"first-page": "165",
"journal-title": "Clin Infect Dis",
"key": "1246_CR26",
"unstructured": "Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and Nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis. 2023;76:165–71.",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1128/aac.00697-22",
"author": "H Mukae",
"doi-asserted-by": "publisher",
"journal-title": "Antimicrob Agents Chemother",
"key": "1246_CR27",
"unstructured": "Mukae H, Yotsuyanagi H, Ohmagari N, et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3c-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother. 2022;66:e0069722.",
"volume": "66",
"year": "2022"
},
{
"author": "K Tanaka",
"first-page": "1711",
"journal-title": "Jpn Pharmacol Ther.",
"key": "1246_CR28",
"unstructured": "Tanaka K, Suzuki A, Kanayama M, et al. The safety evaluation of long-term or excessive intake of the beverage containing Lactococcus lactis subsp. lactis JCM 5805 and resistant maltodextrin—a randomized, double-blind, placebo-controlled, parallel-group trial. Jpn Pharmacol Ther. 2015;43:1711–27.",
"volume": "43",
"year": "2015"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-025-01246-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of Lactococcus lactis Strain Plasma in Patients with Mild COVID-19: A Multicenter, Double-Blinded, Randomized-Controlled Trial (PLATEAU Study)",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy"
}
