Risk of COVID-19 Diagnosis and Hospitalization in Patients with Osteoarthritis or Back Pain Treated with Ibuprofen Compared to Other NSAIDs or Paracetamol: A Network Cohort Study
et al., Drugs, doi:10.1007/s40265-022-01822-z, Jul 2022 (preprint)
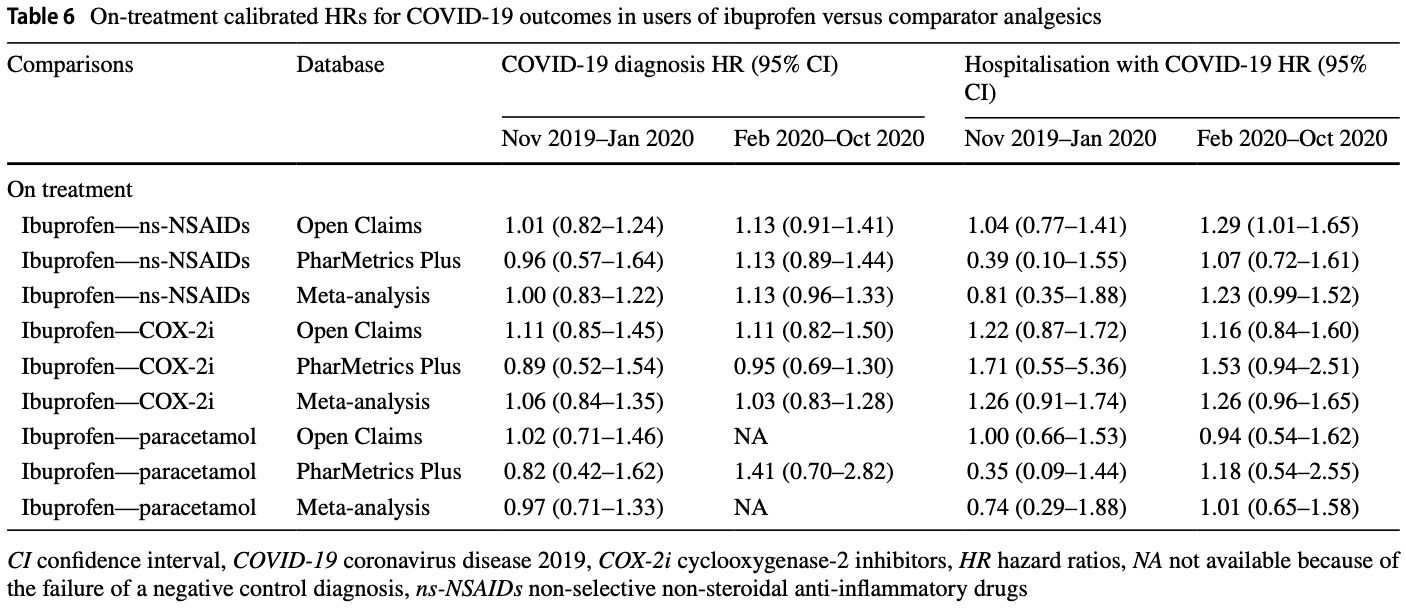
PSM retrospective 1,697,522 osteoarthritis or back pain patients in the US, showing no significant differences in COVID-19 cases and hospitalization for ibuprofen vs. other NSAIDs.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers ibuprofen and acetaminophen.
|
risk of hospitalization, 12.5% higher, HR 1.12, p = 0.26, Open Claims, PharMetrics Plus, both periods combined.
|
|
risk of case, 7.6% higher, HR 1.08, p = 0.25, Open Claims, PharMetrics Plus, both periods combined.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Xie et al., 13 Jul 2022, retrospective, USA, peer-reviewed, 9 authors, study period 1 February, 2020 - 31 October, 2020, this trial compares with another treatment - results may be better when compared to placebo.
Risk of COVID-19 Diagnosis and Hospitalisation in Patients with Osteoarthritis or Back Pain Treated with Ibuprofen Compared to Other NSAIDs or Paracetamol: A Network Cohort Study
Drugs, doi:10.1007/s40265-022-01822-z
Objective We aimed to investigate whether ibuprofen use, compared with other non-selective non-steroidal anti-inflammatory drugs (ns-NSAIDs), cyclooxygenase-2 inhibitors (COX-2i) or paracetamol, increases the risk of coronavirus disease 2019 (COVID-19) diagnosis or hospitalisation. Design A prevalent user and active comparator cohort study. Setting Two US claims databases (Open Claims and PharMetrics Plus) mapped to the Observational Medical Outcomes Partnership Common Data Model. Participants Insured patients with a history of osteoarthritis or back pain and receiving ibuprofen, other ns-NSAIDs, COX-2i or paracetamol between
or paracetamol, was not associated with an increased risk of susceptibility and severity of COVID-19.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s40265-022-01822-z.
Declarations Funding Abbott contracted IQVIA for the conduct of this study. Prof. Prieto-Alhambra receives funding from the UK National Institute for Health Research in the form of a Senior Research Fellowship, and as part of the Oxford National Institute for Health Research Biomedical Research Centre. Prof. Giustino Varrassi had a contract with Abbott International, as a consultant for scientific projects. He is also a consultant for scientific projects with Dompé Farmaceutici and Menarini Group. Ethics Approval These assets are de-identified, commercially available data products that could be purchased and licensed by any researcher. The collection and de-identification of these data assets is a process that is commercial intellectual property and not privileged to the data licensees and the co-authors on this study. Licensees of these data have signed data use agreements with the data vendors that detail the usage protocols for running retrospective research on these databases. All analyses performed in this study were in accordance with data use agreement terms as specified by the data owners. As these data are deemed commercial assets, there is no institutional review board applicable to the usage and dissemination of these result..
References
Au, Reed, Curtis, High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis, Ann Rheum Dis, doi:10.1136/ard.2010.128637
Chandan, Zemedikun, Thayakaran, Nonsteroidal antiinflammatory drugs and susceptibility to COVID-19, Arthritis Rheumatol, doi:10.1002/art.41593
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ, doi:10.1136/bmj.m1086
Drake, Fairfield, Pius, Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(21)00104-1
Esba, Alqahtani, Thomas, Shamas, Alswaidan et al., Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study, Infect Dis Ther, doi:10.1007/s40121-020-00363-w
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Lancet Respir Med, doi:10.1016/S2213-2600(20)30116-8
Fernandez-Gutierrez, Leon, Madrid, Hospital admissions in inflammatory rheumatic diseases during the peak of COVID-19 pandemic: incidence and role of disease-modifying agents, Ther Adv Musculoskelet Dis, doi:10.1177/1759720X20962692
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Kow, Hasan, The risk of mortality in patients with COVID-19 with pre-diagnosis use of NSAIDs: a meta-analysis, Inflammopharmacology, doi:10.1007/s10787-021-00810-1
Levy, Clish, Schmidt, Gronert, Serhan, Lipid mediator class switching during acute inflammation: signals in resolution, Nat Immunol, doi:10.1038/89759
Monti, Montecucco, Non-steroidal anti-inflammatory treatment during COVID-19: friend or foe? Response to: 'Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID)' by Giollo et al, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217638
Moore, Pollack, Butkerait, Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs, Ther Clin Risk Manag, doi:10.2147/TCRM.S79135
Nice, COVID-19 rapid evidence summary: acute use of non-steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19
Ohdsi, OMOP Common Data Model
Park, Lee, You, Kim, Yang, Non-steroidal antiinflammatory agent use may not be associated with mortality of coronavirus disease 19, Sci Rep, doi:10.1038/s41598-021-84539-5
Petersen, Porter, Gruber, Wang, Van Der Laan, Diagnosing and responding to violations in the positivity assumption, Stat Methods Med Res, doi:10.1177/0962280210386207
Prats-Uribe, Sena, Lai, Use of repurposed and adjuvant drugs in hospital patients with COVID-19: multinational network cohort study, BMJ, doi:10.1136/bmj.n1038
Prieto-Alhambra, Kostka, Duarte-Salles, Unraveling COVID-19: a large-scale characterization of 4.5 million COVID-19 cases using CHARYBDIS, Clin Epidemiol, doi:10.2147/CLEP.S323292
Qiao, Wang, Chen, Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats, Cardiology, doi:10.1159/000375362
Roy, Coronavirus: alerte sur l'ibuprofène et autres anti-inflammatoires
Schuemie, Hripcsak, Ryan, Madigan, Suchard, Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data, Proc Natl Acad Sci, doi:10.1073/pnas.1708282114
Schuemie, Hripcsak, Ryan, Madigan, Suchard, Robust empirical calibration of p-values using observational data, Stat Med, doi:10.1002/sim.6977
Serhan, Pro-resolving lipid mediators are leads for resolution physiology, Nature, doi:10.1038/nature13479
Tian, Schuemie, Suchard, Evaluating large-scale propensity score performance through real-world and synthetic data experiments, Int J Epidemiol, doi:10.1093/ije/dyy120
Torjesen, Covid-19: ibuprofen can be used for symptoms, says UK agency, but reasons for change in advice are unclear, BMJ, doi:10.1136/bmj.m1555
Wong, Mackenna, Morton, Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-219517
Woodward, Formulae for sample size, power and minimum detectable relative risk in medical studies, J R Stat Soc D, doi:10.2307/2348252
DOI record:
{
"DOI": "10.1007/s40265-022-01822-z",
"ISSN": [
"0012-6667",
"1179-1950"
],
"URL": "http://dx.doi.org/10.1007/s40265-022-01822-z",
"alternative-id": [
"1822"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "27 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 2,
"value": "24 January 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Funding",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Abbott contracted IQVIA for the conduct of this study. Prof. Prieto-Alhambra receives funding from the UK National Institute for Health Research in the form of a Senior Research Fellowship, and as part of the Oxford National Institute for Health Research Biomedical Research Centre. Prof. Giustino Varrassi had a contract with Abbott International, as a consultant for scientific projects. He is also a consultant for scientific projects with Dompé Farmaceutici and Menarini Group."
},
{
"group": {
"label": "Conflicts of Interest/Competing Interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "All authors have completed the ICMJE disclosure form at ExternalRef removed and declare the following interests: Dani Prieto-Alhambra receives funding from the UK National Institute for Health Research in the form of a senior research fellowship and from the Oxford National Institute for Health Research Biomedical Research Centre. Junqing Xie receives the Clarendon Fund and Jardine scholarship (University of Oxford) to support her DPhil study. Dani Prieto-Alhambra’s research group has received research grants from the European Medicines Agency, the Innovative Medicines Initiative, Amgen, Chiesi and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, AstraZeneca and UCB Biopharma."
},
{
"group": {
"label": "Ethics Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "These assets are de-identified, commercially available data products that could be purchased and licensed by any researcher. The collection and de-identification of these data assets is a process that is commercial intellectual property and not privileged to the data licensees and the co-authors on this study. Licensees of these data have signed data use agreements with the data vendors that detail the usage protocols for running retrospective research on these databases. All analyses performed in this study were in accordance with data use agreement terms as specified by the data owners. As these data are deemed commercial assets, there is no institutional review board applicable to the usage and dissemination of these result sets or required registration of the protocol with additional ethics oversight. Compliance with data use agreement terms, which stipulate how these data can be used and for what purpose, is sufficient for the licensing commercial entities."
},
{
"group": {
"label": "Consent to Participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Not applicable."
},
{
"group": {
"label": "Consent for Publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 6,
"value": "Not applicable."
},
{
"group": {
"label": "Availability of Data and Material",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 7,
"value": "Patient-level data cannot be shared without approval from data custodians owing to local information governance and data protection regulations. Additional summary data, analytical code and detailed definitions of algorithms for identifying the events are available from the corresponding author on reasonable request to access the GitHub repository."
},
{
"group": {
"label": "Code Availability",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 8,
"value": "Not applicable."
},
{
"group": {
"label": "Authors’ Contributors",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 9,
"value": "JQ-X, DP-A, SS, CT and GV conceived the study and contributed to the study design. JQ-X, JB and JA conducted the statistical analyses. JQ-X and DP-A interpreted the results and wrote the manuscript. All authors contributed to writing the manuscript, approved the final version and had final responsibility for the decision to submit for publication. The lead authors (JQ-X and JB) affirm that this manuscript is an honest, accurate and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. DP-A is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0040-0042",
"affiliation": [],
"authenticated-orcid": false,
"family": "Xie",
"given": "Junqing",
"sequence": "first"
},
{
"affiliation": [],
"family": "Brash",
"given": "James T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turkmen",
"given": "Cigdem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Driessen",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Varrassi",
"given": "Giustino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Argyriou",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seager",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reich",
"given": "Christian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3950-6346",
"affiliation": [],
"authenticated-orcid": false,
"family": "Prieto-Alhambra",
"given": "Daniel",
"sequence": "additional"
}
],
"container-title": "Drugs",
"container-title-short": "Drugs",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T12:34:35Z",
"timestamp": 1674563675000
},
"deposited": {
"date-parts": [
[
2023,
2,
15
]
],
"date-time": "2023-02-15T07:30:28Z",
"timestamp": 1676446228000
},
"funder": [
{
"DOI": "10.13039/501100000272",
"doi-asserted-by": "publisher",
"name": "National Institute for Health and Care Research"
},
{
"name": "Oxford Clarendon Foundation"
},
{
"name": "Abbott contracted IQVIA for the conducting of this study"
},
{
"name": "Had a contract with Abbott International, as Consultant for scientific projects"
}
],
"indexed": {
"date-parts": [
[
2023,
9,
20
]
],
"date-time": "2023-09-20T16:19:00Z",
"timestamp": 1695226740625
},
"is-referenced-by-count": 4,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
1,
24
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2023,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40265-022-01822-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40265-022-01822-z/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40265-022-01822-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "249-263",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
1,
24
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
2
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1136/bmj.m1086",
"author": "M Day",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1822_CR1",
"unstructured": "Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368: m1086. https://doi.org/10.1136/bmj.m1086.",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1555",
"author": "I Torjesen",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1822_CR2",
"unstructured": "Torjesen I. Covid-19: ibuprofen can be used for symptoms, says UK agency, but reasons for change in advice are unclear. BMJ. 2020;369: m1555. https://doi.org/10.1136/bmj.m1555.",
"volume": "369",
"year": "2020"
},
{
"key": "1822_CR3",
"unstructured": "Roy S. Coronavirus: alerte sur l’ibuprofène et autres anti-inflammatoires. Le Figaro. Updated March 14, 2020. 2022. https://www.lefigaro.fr/sciences/coronavirus-alerte-sur-l-ibuprofene-et-autres-anti-inflammatoires-20200314. Accessed 28 Feb 2022."
},
{
"DOI": "10.1177/1759720X20962692",
"doi-asserted-by": "publisher",
"key": "1822_CR4",
"unstructured": "Fernandez-Gutierrez B, Leon L, Madrid A, et al. Hospital admissions in inflammatory rheumatic diseases during the peak of COVID-19 pandemic: incidence and role of disease-modifying agents. Ther Adv Musculoskelet Dis. 2021. doi: https://doi.org/10.1177/1759720X20962692."
},
{
"DOI": "10.1016/S2213-2600(20)30116-8",
"author": "L Fang",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Lancet Respir Med",
"key": "1822_CR5",
"unstructured": "Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4): e21. https://doi.org/10.1016/S2213-2600(20)30116-8.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "1822_CR6",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-80.e8. https://doi.org/10.1016/j.cell.2020.02.052.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1136/ard.2010.128637",
"author": "K Au",
"doi-asserted-by": "publisher",
"first-page": "785",
"issue": "5",
"journal-title": "Ann Rheum Dis",
"key": "1822_CR7",
"unstructured": "Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–91. https://doi.org/10.1136/ard.2010.128637.",
"volume": "70",
"year": "2011"
},
{
"key": "1822_CR8",
"unstructured": "European Medicines Agency. EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19. 2020. https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19. Accessed 5 Jul 2020 ."
},
{
"DOI": "10.1136/annrheumdis-2020-217638",
"doi-asserted-by": "publisher",
"key": "1822_CR9",
"unstructured": "Monti S, Montecucco C. Non-steroidal anti-inflammatory treatment during COVID-19: friend or foe? Response to: 'Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID)' by Giollo et al. Ann Rheum Dis. 2021;80(2):e13.https://doi.org/10.1136/annrheumdis-2020-217638"
},
{
"key": "1822_CR10",
"unstructured": "NICE. Key messages. COVID-19 rapid evidence summary: acute use of non-steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. 2020. https://www.nice.org.uk/advice/es23/chapter/Key-messages. Accessed 5 July 2020."
},
{
"DOI": "10.1002/art.41593",
"author": "JS Chandan",
"doi-asserted-by": "publisher",
"first-page": "731",
"issue": "5",
"journal-title": "Arthritis Rheumatol",
"key": "1822_CR11",
"unstructured": "Chandan JS, Zemedikun DT, Thayakaran R, et al. Nonsteroidal antiinflammatory drugs and susceptibility to COVID-19. Arthritis Rheumatol. 2021;73(5):731–9. https://doi.org/10.1002/art.41593.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2020-219517",
"author": "AY Wong",
"doi-asserted-by": "publisher",
"first-page": "943",
"issue": "7",
"journal-title": "Ann Rheum Dis",
"key": "1822_CR12",
"unstructured": "Wong AY, MacKenna B, Morton CE, et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80(7):943–51. https://doi.org/10.1136/annrheumdis-2020-219517.",
"volume": "80",
"year": "2021"
},
{
"key": "1822_CR13",
"unstructured": "OHDSI. OMOP Common Data Model. 2021. https://www.ohdsi.org/data-standardization/the-common-data-model/. Accessed 7 Oct 2021."
},
{
"DOI": "10.2147/CLEP.S323292",
"author": "D Prieto-Alhambra",
"doi-asserted-by": "publisher",
"first-page": "369",
"journal-title": "Clin Epidemiol.",
"key": "1822_CR14",
"unstructured": "Prieto-Alhambra D, Kostka K, Duarte-Salles T, et al. Unraveling COVID-19: a large-scale characterization of 4.5 million COVID-19 cases using CHARYBDIS. Clin Epidemiol. 2022;14:369–84. https://doi.org/10.2147/CLEP.S323292.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1136/bmj.n1038",
"author": "A Prats-Uribe",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1822_CR15",
"unstructured": "Prats-Uribe A, Sena AG, Lai LYH, et al. Use of repurposed and adjuvant drugs in hospital patients with COVID-19: multinational network cohort study. BMJ. 2021;373: n1038. https://doi.org/10.1136/bmj.n1038.",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.2307/2348252",
"author": "M Woodward",
"doi-asserted-by": "publisher",
"first-page": "185",
"issue": "2",
"journal-title": "J R Stat Soc D.",
"key": "1822_CR16",
"unstructured": "Woodward M. Formulae for sample size, power and minimum detectable relative risk in medical studies. J R Stat Soc D. 1992;41(2):185. https://doi.org/10.2307/2348252.",
"volume": "41",
"year": "1992"
},
{
"DOI": "10.1093/ije/dyy120",
"author": "Y Tian",
"doi-asserted-by": "publisher",
"first-page": "2005",
"issue": "6",
"journal-title": "Int J Epidemiol",
"key": "1822_CR17",
"unstructured": "Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005–14. https://doi.org/10.1093/ije/dyy120.",
"volume": "47",
"year": "2018"
},
{
"DOI": "10.1002/sim.6977",
"author": "MJ Schuemie",
"doi-asserted-by": "publisher",
"first-page": "3883",
"issue": "22",
"journal-title": "Stat Med",
"key": "1822_CR18",
"unstructured": "Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Robust empirical calibration of p-values using observational data. Stat Med. 2016;35(22):3883–8. https://doi.org/10.1002/sim.6977.",
"volume": "35",
"year": "2016"
},
{
"DOI": "10.1073/pnas.1708282114",
"author": "MJ Schuemie",
"doi-asserted-by": "publisher",
"first-page": "2571",
"issue": "11",
"journal-title": "Proc Natl Acad Sci USA",
"key": "1822_CR19",
"unstructured": "Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci USA. 2018;115(11):2571–7. https://doi.org/10.1073/pnas.1708282114.",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1177/0962280210386207",
"author": "ML Petersen",
"doi-asserted-by": "publisher",
"first-page": "31",
"issue": "1",
"journal-title": "Stat Methods Med Res",
"key": "1822_CR20",
"unstructured": "Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res. 2012;21(1):31–54. https://doi.org/10.1177/0962280210386207.",
"volume": "21",
"year": "2012"
},
{
"DOI": "10.1038/nature13479",
"author": "CN Serhan",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "7503",
"journal-title": "Nature",
"key": "1822_CR21",
"unstructured": "Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. https://doi.org/10.1038/nature13479.",
"volume": "510",
"year": "2014"
},
{
"DOI": "10.1038/89759",
"author": "BD Levy",
"doi-asserted-by": "publisher",
"first-page": "612",
"issue": "7",
"journal-title": "Nat Immunol",
"key": "1822_CR22",
"unstructured": "Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–9. https://doi.org/10.1038/89759.",
"volume": "2",
"year": "2001"
},
{
"DOI": "10.1159/000375362",
"author": "W Qiao",
"doi-asserted-by": "publisher",
"first-page": "97",
"issue": "2",
"journal-title": "Cardiology",
"key": "1822_CR23",
"unstructured": "Qiao W, Wang C, Chen B, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131(2):97–106. https://doi.org/10.1159/000375362.",
"volume": "131",
"year": "2015"
},
{
"DOI": "10.1007/s40121-020-00363-w",
"author": "LC Abu Esba",
"doi-asserted-by": "publisher",
"first-page": "253",
"issue": "1",
"journal-title": "Infect Dis Ther",
"key": "1822_CR24",
"unstructured": "Abu Esba LC, Alqahtani RA, Thomas A, Shamas N, Alswaidan L, Mardawi G. Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2021;10(1):253–68. https://doi.org/10.1007/s40121-020-00363-w.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(21)00104-1",
"author": "TM Drake",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Rheumatol",
"key": "1822_CR25",
"unstructured": "Drake TM, Fairfield CJ, Pius R, et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021. https://doi.org/10.1016/S2665-9913(21)00104-1.",
"year": "2021"
},
{
"DOI": "10.1007/s10787-021-00810-1",
"author": "CS Kow",
"doi-asserted-by": "publisher",
"first-page": "641",
"issue": "3",
"journal-title": "Inflammopharmacology",
"key": "1822_CR26",
"unstructured": "Kow CS, Hasan SS. The risk of mortality in patients with COVID-19 with pre-diagnosis use of NSAIDs: a meta-analysis. Inflammopharmacology. 2021;29(3):641–4. https://doi.org/10.1007/s10787-021-00810-1.",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-84539-5",
"author": "J Park",
"doi-asserted-by": "publisher",
"first-page": "5087",
"issue": "1",
"journal-title": "Sci Rep",
"key": "1822_CR27",
"unstructured": "Park J, Lee S-H, You SC, Kim J, Yang K. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci Rep. 2021;11(1):5087. https://doi.org/10.1038/s41598-021-84539-5.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.2147/TCRM.S79135",
"author": "N Moore",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "Ther Clin Risk Manag",
"key": "1822_CR28",
"unstructured": "Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–75. https://doi.org/10.2147/TCRM.S79135.",
"volume": "11",
"year": "2015"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40265-022-01822-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)"
],
"subtitle": [],
"title": "Risk of COVID-19 Diagnosis and Hospitalisation in Patients with Osteoarthritis or Back Pain Treated with Ibuprofen Compared to Other NSAIDs or Paracetamol: A Network Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "83"
}
xie