Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population
et al., JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044, Jan 2024
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Analysis of Medicare beneficiaries in 2022 showing that outpatient COVID-19 treatments like antivirals and monoclonal antibodies were disproportionately used by patients at lower risk of severe infection and outcomes.
Retrospective studies of treatment effectiveness may overestimate efficacy if they do not fully adjust for this difference. Full adjustment may not be possible because potentially related factors are typically not available in the data. For example, patients with greater knowledge of effective treatments may be more likely to access prescription treatments but result in confounding because they are also more likely to use known beneficial non-prescription treatments.
Study covers molnupiravir, paxlovid, remdesivir, bamlanivimab/etesevimab, casirivimab/imdevimab, sotrovimab, and bebtelovimab.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
Wilcock et al., 26 Jan 2024, retrospective, peer-reviewed, 8 authors, study period January 2020 - December 2022.
Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population
JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044
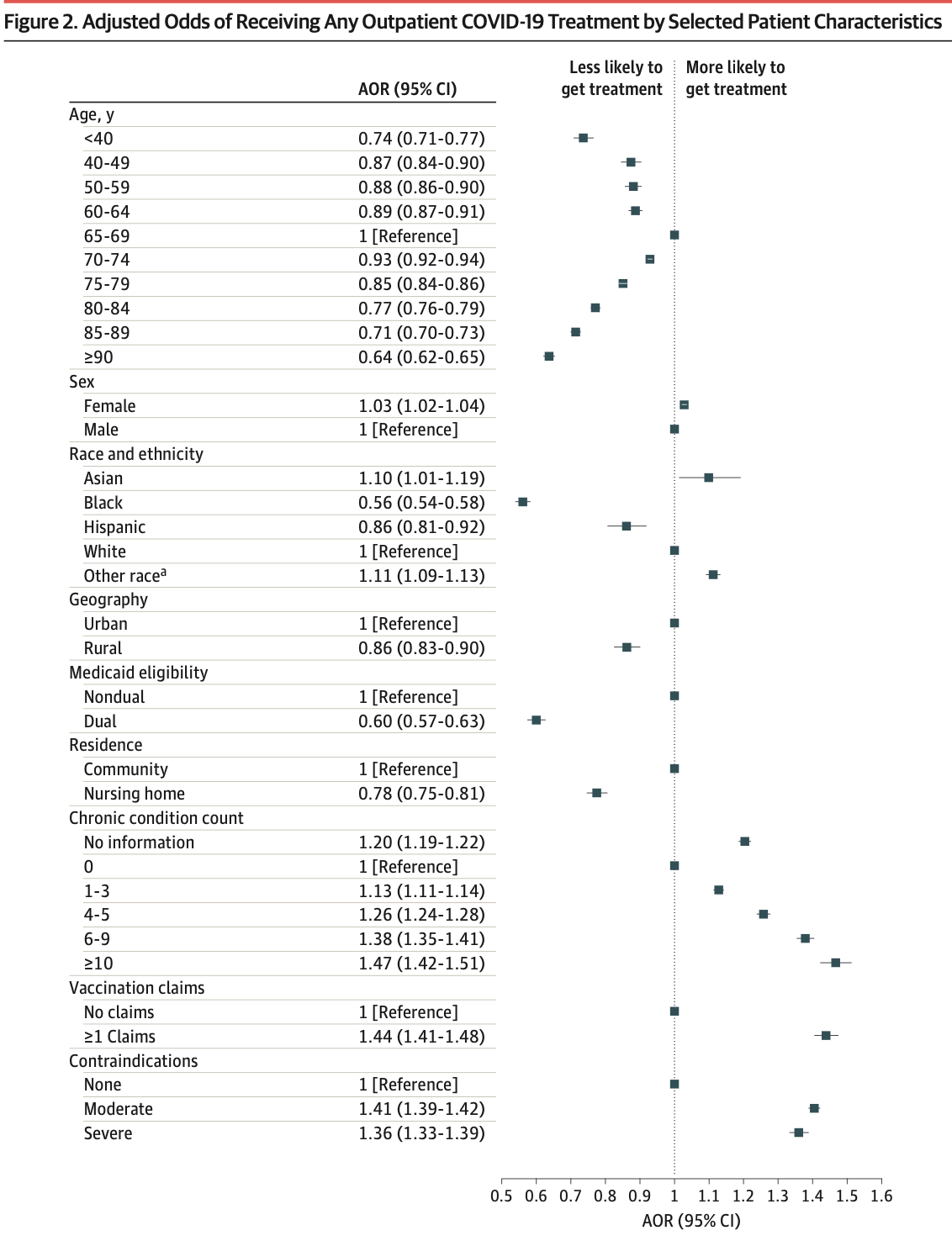
IMPORTANCE Multiple therapies are available for outpatient treatment of COVID-19 that are highly effective at preventing hospitalization and mortality. Although racial and socioeconomic disparities in use of these therapies have been documented, limited evidence exists on what factors explain differences in use and the potential public health relevance of these differences. OBJECTIVE To assess COVID-19 outpatient treatment utilization in the Medicare population and simulate the potential outcome of allocating treatment according to patient risk for severe COVID-19. DESIGN, SETTING, AND PARTICIPANTS This cross-sectional study included patients enrolled in Medicare in 2022 across the US, identified with 100% Medicare fee-for-service claims. MAIN OUTCOMES AND MEASURES The primary outcome was any COVID-19 outpatient therapy utilization. Secondary outcomes included COVID-19 testing, ambulatory visits, and hospitalization. Differences in outcomes were estimated based on patient demographics, treatment contraindications, and a composite risk score for mortality after COVID-19 based on demographics and comorbidities. A simulation of reallocating COVID-19 treatment, particularly with nirmatrelvir, to those at high risk of severe disease was performed, and the potential COVID-19 hospitalizations and mortality outcomes were assessed. RESULTS In 2022, 6.0% of 20 026 910 beneficiaries received outpatient COVID-19 treatment, 40.5% of which had no associated COVID-19 diagnosis within 10 days. Patients with higher risk for severe disease received less outpatient treatment, such as 6.4% of those aged 65 to 69 years compared with 4.9% of those 90 years and older (adjusted odds ratio [aOR], 0.64 [95% CI, 0.62-0.65]) and 6.4% of White patients compared with 3.0% of Black patients (aOR, 0.56 [95% CI, 0.54-0.58]). In the highest COVID-19 severity risk quintile, 2.6% were hospitalized for COVID-19 and 4.9% received outpatient treatment, compared with 0.2% and 7.5% in the lowest quintile. These patterns were similar among patients with a documented COVID-19 diagnosis, those with no claims for vaccination, and patients who are insured with Medicare Advantage. Differences were not explained by variable COVID-19 testing, ambulatory visits, or treatment contraindications. Reallocation of 2022 outpatient COVID-19 treatment, particularly with nirmatrelvir, based on risk for severe COVID-19 would have averted 16 503 COVID-19 deaths (16.3%) in the sample. CONCLUSION In this cross-sectional study, outpatient COVID-19 treatment was disproportionately accessed by beneficiaries at lower risk for severe infection, undermining its potential public health benefit. Undertreatment was not driven by lack of clinical access or treatment contraindications.
References
Abraham, Nohria, Neilan, Cardiovascular drug interactions with nirmatrelvir/ritonavir in patients with COVID-19: JACC review topic of the week, J Am Coll Cardiol, doi:10.1016/j.jacc.2022.08.800
Aggarwal, Molina, Beaty, Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00011-7
Arbel, Sagy, Hoshen, Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N Engl J Med, doi:10.1056/NEJMoa2204919
Bajema, Berry, Streja, Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among US veterans: target trial emulation studies with one-month and six-month outcomes, Ann Intern Med, doi:10.7326/M22-3565
Barnett, Bitton, Souza, Landon, Trends in outpatient care for Medicare beneficiaries and implications for primary care, 2000 to 2019, Ann Intern Med, doi:10.7326/M21-1523
Behr, Maddox, Epstein, Orav, Barnett, Anti-SARS-CoV-2 monoclonal antibody distribution to high-risk Medicare beneficiaries, 2020-2021, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2022.1243&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamahealthforum.2023.5044
Beleche, Bush, Finegold, Understanding coverage considerations for COVID-19 vaccines and treatments
Berenbrok, Tang, Gabriel, Access to community pharmacies: a nationwide geographic information systems cross-sectional analysis, J Am Pharm Assoc, doi:10.1016/j.japh.2022.07.003
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Boehmer, Koumans, Skillen, Racial and ethnic disparities in outpatient treatment of COVID-19-United States, January-July 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7143a2
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 in a large US health system: a population-based cohort study, Ann Intern Med, doi:10.7326/M22-2141
Gold, Kelleher, Magid, Dispensing of oral antiviral drugs for treatment of COVID-19 by zip codelevel social vulnerability-United States, December 23, 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7125e1
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hooper, Nápoles, Ej, COVID-19 and racial/ethnic disparities, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.8598&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamahealthforum.2023.5044
Intrator, Hiris, Berg, Miller, Mor, The residential history file: studying nursing home residents' longterm care histories, Health Serv Res, doi:10.1111/j.1475-6773.2010.01194.x
Jarrín, Nyandege, Grafova, Dong, Lin, Validity of race and ethnicity codes in Medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits, Med Care, doi:10.1097/MLR.0000000000001216
Luisi, Sullivan, Sanchez, Use of COVIDTests.gov at-home test kits among adults in a national household probability sample-United States, 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7216a6
Murphy, Samson, Sommers, COVID-19 antivirals utilization: geographic and demographic patterns of treatment in 2022
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Patel, Mehrotra, Huskamp, Uscher-Pines, Ganguli et al., Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US, JAMA Intern Med, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2020.5928&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamahealthforum.2023.5044
Patel, Mehrotra, Huskamp, Uscher-Pines, Ganguli et al., Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States, Health Aff (Millwood), doi:10.1377/hlthaff.2020.01786
Patel, Rose, Barnett, Huskamp, Uscher-Pines et al., Community factors associated with telemedicine use during the COVID-19 pandemic, JAMA Netw Open, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jamanetworkopen.2021.10330&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamahealthforum.2023.5044
Silk, Scobie, Duck, COVID-19 surveillance after expiration of the public health emergency declaration-United States, May 11, 2023, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7219e1
Sullivan, Perrine, Kelleher, Notes from the field: dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability-United States, December 23, 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7143a3
Tarabichi, Kaelber, Thornton, Early racial and ethnic disparities in the prescription of nirmatrelvir for COVID-19, J Gen Intern Med, doi:10.1007/s11606-022-07844-3
Wiltz, Feehan, Molinari, Racial and ethnic disparities in receipt of medications for treatment of COVID-19-United States, March 2020-August 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7103e1
DOI record:
{
"DOI": "10.1001/jamahealthforum.2023.5044",
"ISSN": [
"2689-0186"
],
"URL": "http://dx.doi.org/10.1001/jamahealthforum.2023.5044",
"abstract": "<jats:sec id=\"ab-aoi230094-4\"><jats:title>Importance</jats:title><jats:p>Multiple therapies are available for outpatient treatment of COVID-19 that are highly effective at preventing hospitalization and mortality. Although racial and socioeconomic disparities in use of these therapies have been documented, limited evidence exists on what factors explain differences in use and the potential public health relevance of these differences.</jats:p></jats:sec><jats:sec id=\"ab-aoi230094-5\"><jats:title>Objective</jats:title><jats:p>To assess COVID-19 outpatient treatment utilization in the Medicare population and simulate the potential outcome of allocating treatment according to patient risk for severe COVID-19.</jats:p></jats:sec><jats:sec id=\"ab-aoi230094-6\"><jats:title>Design, Setting, and Participants</jats:title><jats:p>This cross-sectional study included patients enrolled in Medicare in 2022 across the US, identified with 100% Medicare fee-for-service claims.</jats:p></jats:sec><jats:sec id=\"ab-aoi230094-7\"><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was any COVID-19 outpatient therapy utilization. Secondary outcomes included COVID-19 testing, ambulatory visits, and hospitalization. Differences in outcomes were estimated based on patient demographics, treatment contraindications, and a composite risk score for mortality after COVID-19 based on demographics and comorbidities. A simulation of reallocating COVID-19 treatment, particularly with nirmatrelvir, to those at high risk of severe disease was performed, and the potential COVID-19 hospitalizations and mortality outcomes were assessed.</jats:p></jats:sec><jats:sec id=\"ab-aoi230094-8\"><jats:title>Results</jats:title><jats:p>In 2022, 6.0% of 20 026 910 beneficiaries received outpatient COVID-19 treatment, 40.5% of which had no associated COVID-19 diagnosis within 10 days. Patients with higher risk for severe disease received less outpatient treatment, such as 6.4% of those aged 65 to 69 years compared with 4.9% of those 90 years and older (adjusted odds ratio [aOR], 0.64 [95% CI, 0.62-0.65]) and 6.4% of White patients compared with 3.0% of Black patients (aOR, 0.56 [95% CI, 0.54-0.58]). In the highest COVID-19 severity risk quintile, 2.6% were hospitalized for COVID-19 and 4.9% received outpatient treatment, compared with 0.2% and 7.5% in the lowest quintile. These patterns were similar among patients with a documented COVID-19 diagnosis, those with no claims for vaccination, and patients who are insured with Medicare Advantage. Differences were not explained by variable COVID-19 testing, ambulatory visits, or treatment contraindications. Reallocation of 2022 outpatient COVID-19 treatment, particularly with nirmatrelvir, based on risk for severe COVID-19 would have averted 16 503 COVID-19 deaths (16.3%) in the sample.</jats:p></jats:sec><jats:sec id=\"ab-aoi230094-9\"><jats:title>Conclusion</jats:title><jats:p>In this cross-sectional study, outpatient COVID-19 treatment was disproportionately accessed by beneficiaries at lower risk for severe infection, undermining its potential public health benefit. Undertreatment was not driven by lack of clinical access or treatment contraindications.</jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Harvard Medical School, Boston, Massachusetts"
}
],
"family": "Wilcock",
"given": "Andrew D.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health, Boston, Massachusetts"
}
],
"family": "Kissler",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School, Boston, Massachusetts"
},
{
"name": "Beth Israel Deaconess Medical Center, Boston, Massachusetts"
}
],
"family": "Mehrotra",
"given": "Ateev",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Rochester Medical Center, Rochester, New York"
}
],
"family": "McGarry",
"given": "Brian E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health, Boston, Massachusetts"
}
],
"family": "Sommers",
"given": "Benjamin D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School, Boston, Massachusetts"
}
],
"family": "Grabowski",
"given": "David C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health, Boston, Massachusetts"
}
],
"family": "Grad",
"given": "Yonatan H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health, Boston, Massachusetts"
},
{
"name": "Division of General Internal Medicine and Primary Care, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts"
}
],
"family": "Barnett",
"given": "Michael L.",
"sequence": "additional"
}
],
"container-title": "JAMA Health Forum",
"container-title-short": "JAMA Health Forum",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
26
]
],
"date-time": "2024-01-26T16:30:54Z",
"timestamp": 1706286654000
},
"deposited": {
"date-parts": [
[
2024,
1,
26
]
],
"date-time": "2024-01-26T17:00:41Z",
"timestamp": 1706288441000
},
"indexed": {
"date-parts": [
[
2024,
1,
27
]
],
"date-time": "2024-01-27T00:03:18Z",
"timestamp": 1706313798695
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
26
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2024,
1,
5
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama-health-forum/articlepdf/2814359/wilcock_2024_oi_230094_1706287370.16897.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e235044",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2024,
1,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
26
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "aoi230094r2",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients.",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "aoi230094r3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7103e1",
"article-title": "Racial and ethnic disparities in receipt of medications for treatment of COVID-19—United States, March 2020-August 2021.",
"author": "Wiltz",
"doi-asserted-by": "publisher",
"first-page": "96",
"issue": "3",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "aoi230094r4",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.1243",
"article-title": "Anti-SARS-CoV-2 monoclonal antibody distribution to high-risk Medicare beneficiaries, 2020-2021.",
"author": "Behr",
"doi-asserted-by": "publisher",
"first-page": "980",
"issue": "10",
"journal-title": "JAMA",
"key": "aoi230094r5",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/j.japh.2022.07.003",
"article-title": "Access to community pharmacies: a nationwide geographic information systems cross-sectional analysis.",
"author": "Berenbrok",
"doi-asserted-by": "publisher",
"first-page": "1816",
"issue": "6",
"journal-title": "J Am Pharm Assoc (2003)",
"key": "aoi230094r8",
"volume": "62",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7143a2",
"article-title": "Racial and ethnic disparities in outpatient treatment of COVID-19—United States, January-July 2022.",
"author": "Boehmer",
"doi-asserted-by": "publisher",
"first-page": "1359",
"issue": "43",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "aoi230094r9",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1007/s11606-022-07844-3",
"article-title": "Early racial and ethnic disparities in the prescription of nirmatrelvir for COVID-19.",
"author": "Tarabichi",
"doi-asserted-by": "publisher",
"first-page": "1329",
"issue": "5",
"journal-title": "J Gen Intern Med",
"key": "aoi230094r12",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7125e1",
"article-title": "Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code—level social vulnerability—United States, December 23, 2021-May 21, 2022.",
"author": "Gold",
"doi-asserted-by": "publisher",
"first-page": "825",
"issue": "25",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "aoi230094r13",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7143a3",
"article-title": "Notes from the field: dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability—United States, December 23, 2021-August 28, 2022.",
"author": "Sullivan",
"doi-asserted-by": "publisher",
"first-page": "1384",
"issue": "43",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "aoi230094r14",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.8598",
"article-title": "COVID-19 and racial/ethnic disparities.",
"author": "Webb Hooper",
"doi-asserted-by": "publisher",
"first-page": "2466",
"issue": "24",
"journal-title": "JAMA",
"key": "aoi230094r15",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm7216a6",
"article-title": "Use of COVIDTests.gov at-home test kits among adults in a national household probability sample—United States, 2022.",
"author": "Luisi",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "16",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "aoi230094r16",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7219e1",
"article-title": "COVID-19 surveillance after expiration of the public health emergency declaration—United States, May 11, 2023.",
"author": "Silk",
"doi-asserted-by": "publisher",
"first-page": "523",
"issue": "19",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "aoi230094r18",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1097/MLR.0000000000001216",
"article-title": "Validity of race and ethnicity codes in Medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits.",
"author": "Jarrín",
"doi-asserted-by": "publisher",
"first-page": "e1",
"issue": "1",
"journal-title": "Med Care",
"key": "aoi230094r22",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1111/hesr.2010.46.issue-1p1",
"article-title": "The residential history file: studying nursing home residents' long-term care histories.",
"author": "Intrator",
"doi-asserted-by": "publisher",
"first-page": "120",
"issue": "1p1",
"journal-title": "Health Serv Res",
"key": "aoi230094r23",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.1377/hlthaff.2020.01786",
"article-title": "Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States.",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "349",
"issue": "2",
"journal-title": "Health Aff (Millwood)",
"key": "aoi230094r24",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.5928",
"article-title": "Trends in outpatient care delivery and telemedicine during the COVID-19 pandemic in the US.",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "388",
"issue": "3",
"journal-title": "JAMA Intern Med",
"key": "aoi230094r25",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.10330",
"article-title": "Community factors associated with telemedicine use during the COVID-19 pandemic.",
"author": "Patel",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "JAMA Netw Open",
"key": "aoi230094r26",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.7326/M21-1523",
"article-title": "Trends in outpatient care for Medicare beneficiaries and implications for primary care, 2000 to 2019.",
"author": "Barnett",
"doi-asserted-by": "publisher",
"first-page": "1658",
"issue": "12",
"journal-title": "Ann Intern Med",
"key": "aoi230094r27",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1016/j.jacc.2022.08.800",
"article-title": "Cardiovascular drug interactions with nirmatrelvir/ritonavir in patients with COVID-19: JACC review topic of the week.",
"author": "Abraham",
"doi-asserted-by": "publisher",
"first-page": "1912",
"issue": "20",
"journal-title": "J Am Coll Cardiol",
"key": "aoi230094r28",
"volume": "80",
"year": "2022"
},
{
"DOI": "10.7326/M22-3565",
"article-title": "Effectiveness of COVID-19 treatment with nirmatrelvir-ritonavir or molnupiravir among US veterans: target trial emulation studies with one-month and six-month outcomes.",
"author": "Bajema",
"doi-asserted-by": "publisher",
"first-page": "807",
"issue": "6",
"journal-title": "Ann Intern Med",
"key": "aoi230094r30",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.7326/M22-2141",
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 in a large US health system: a population-based cohort study.",
"author": "Dryden-Peterson",
"doi-asserted-by": "publisher",
"first-page": "77",
"issue": "1",
"journal-title": "Ann Intern Med",
"key": "aoi230094r31",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge.",
"author": "Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "aoi230094r32",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00011-7",
"article-title": "Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study.",
"author": "Aggarwal",
"doi-asserted-by": "publisher",
"first-page": "696",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "aoi230094r33",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients.",
"author": "Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "aoi230094r34",
"volume": "76",
"year": "2023"
},
{
"key": "aoi230094r1",
"unstructured": "National Institutes of Health. Nonhospitalized adults—therapeutic management. COVID-19 Treatment Guidelines. Accessed May 1, 2023. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/"
},
{
"key": "aoi230094r6",
"unstructured": "Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. US Food and Drug Administration. 2021. May 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19"
},
{
"key": "aoi230094r7",
"unstructured": "Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults. FDA. 2021 May 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain"
},
{
"key": "aoi230094r10",
"unstructured": "COVID-19 antivirals utilization: geographic and demographic patterns of treatment in 2022. ASPE. Accessed April 10, 2023. https://aspe.hhs.gov/reports/covid-19-antivirals-utilization"
},
{
"key": "aoi230094r11",
"unstructured": "FDA takes actions to expand use of treatment for outpatients with mild-to-moderate COVID-19. US Food and Drug Administration. 2022. Accessed November 14, 2023. https://www.fda.gov/news-events/press-announcements/fda-takes-actions-expand-use-treatment-outpatients-mild-moderate-covid-19"
},
{
"key": "aoi230094r17",
"unstructured": "COVID data tracker. Centers for Disease Control and Prevention. 2020. Accessed March 8, 2023. https://covid.cdc.gov/covid-data-tracker"
},
{
"key": "aoi230094r19",
"unstructured": "COVID-19 information and resources. Harvard Pilgrim Health Care. Accessed April 10, 2023. https://www.point32health.org/provider/news-center/coronavirus-covid-19-updates-for-providers/"
},
{
"key": "aoi230094r20",
"unstructured": "UW RHRC Rural Urban Commuting Area Codes. UW RUCA. Accessed May 5, 2023. https://depts.washington.edu/uwruca/"
},
{
"key": "aoi230094r21",
"unstructured": "Chronic conditions. Chronic Conditions Data Warehouse. Accessed December 11, 2022. https://www2.ccwdata.org/web/guest/condition-categories-chronic"
},
{
"key": "aoi230094r29",
"unstructured": "Paxlovid drug-drug interactions. COVID-19 Treatment Guidelines. Accessed April 10, 2023. Updated November 2, 2023. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/"
},
{
"key": "aoi230094r35",
"unstructured": "Murphy? SJ, Samson? LW, Sommers? BD. COVID-19 antivirals utilization: geographic and demographic patterns of treatment in 2022. Accessed December 13, 2023. https://aspe.hhs.gov/reports/covid-19-antivirals-utilization"
},
{
"key": "aoi230094r36",
"unstructured": "Beleche? T, Bush? L, Finegold? K, Understanding coverage considerations for COVID-19 vaccines and treatments. 2022. https://aspe.hhs.gov/reports/covid-19-vaccines-treatments"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama-health-forum/fullarticle/2814359"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Public Health, Environmental and Occupational Health",
"Health Policy"
],
"subtitle": [],
"title": "Clinical Risk and Outpatient Therapy Utilization for COVID-19 in the Medicare Population",
"type": "journal-article",
"volume": "5"
}
wilcock
