Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron)
et al., bioRxiv, doi:10.1101/2021.12.19.473354, Dec 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
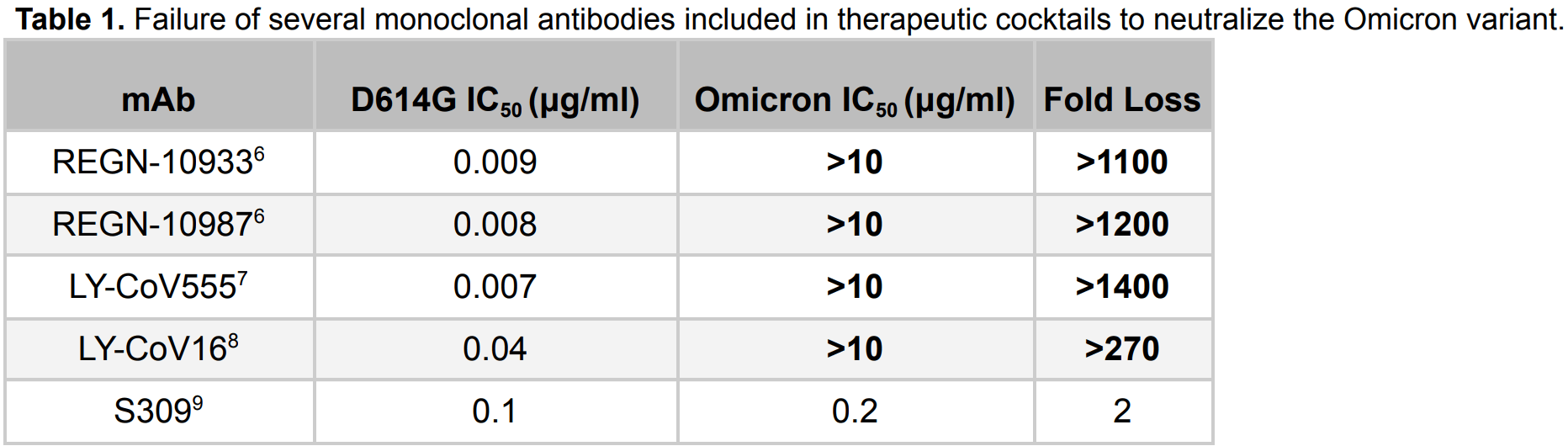
In vitro study showing that omicron is substantially resistant to neutralization by monoclonal antibodies REGN10933, REGN10987, Ly-CoV016 and Ly-CoV555. S309 (the parent of Sotrovimab) had only 2-fold loss in potency.
Study covers casirivimab/imdevimab, bamlanivimab/etesevimab, and sotrovimab.
Sheward et al., 20 Dec 2021, preprint, 11 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron)
doi:10.1101/2021.12.19.473354
The recently-emerged SARS-CoV-2 B.1.1.529 variant (Omicron) is spreading rapidly in many countries, with a spike that is highly diverged from the pandemic founder, raising fears that it may evade neutralizing antibody responses. We cloned the Omicron spike from a diagnostic sample which allowed us to rapidly establish an Omicron pseudotyped virus neutralization assay, sharing initial neutralization results only 13 days after the variant was first reported to the WHO, 8 days after receiving the sample. Here we show that Omicron is substantially resistant to neutralization by several monoclonal antibodies that form part of clinical cocktails. Further, we find neutralizing antibody responses in pooled reference sera sampled shortly after infection or vaccination are substantially less potent against Omicron, with neutralizing antibody titers reduced by up to 45 fold compared to those for the pandemic founder. Similarly, in a cohort of convalescent sera prior to vaccination, neutralization of Omicron was low to undetectable. However, in recent samples from two cohorts from Stockholm, Sweden, antibody responses capable of cross-neutralizing Omicron were prevalent. Sera from infected-then-vaccinated healthcare workers exhibited robust cross-neutralization of Omicron, with an average potency reduction of only 5-fold relative to the pandemic founder variant, and some donors showing no loss at all. A similar pattern was observed in randomly sampled recent blood donors, with an average 7-fold loss of potency. Both cohorts showed substantial between-donor heterogeneity in their ability to neutralize Omicron. Together, these data highlight the extensive but incomplete evasion of neutralizing antibody responses by the Omicron variant, and suggest that increasing the magnitude of neutralizing antibody responses by boosting with unmodified vaccines may suffice to raise titers to levels that are protective.
Author Contributions
Methods
Ethics statement HW and Convalescent cohorts: Informed consent was obtained from all participants as part of an ethics approval (Decision number 2020-01620, with amendments 2020-02881 and 2020-05630) from the Swedish Ethical Review Authority. BD cohort and the Omicron-positive sample from which the spike was cloned were anonymized, and not subject to ethical approvals, as per advisory statement 2020-01807 from the Swedish Ethical Review Authority.
Donor sample description Two cohorts were studied. Cohort 1 comprised serum samples with detectable neutralization against the Wu-Hu-1 founder variant from 17 anonymized blood donors ("BD"), donated during week 48, 2021, in Stockholm, Sweden. Cohort 2 comprised 17 serum samples from Hospital Workers ("HW") at the Karolinska University Hospital in Stockholm 20 , who were invited to participate in a study that aimed to characterize their antibody responses following SARS-CoV-2 infection and subsequent vaccinations. Participants, confirmed PCR positive, had serum sampled in April/May 2020, in June/July 2020 ("convalescent", prior to vaccination), and again in November 2021 ("HW"). Convalescent samples are from 9 unique donors, with 3 donors sampled in both April/May 2020 and June/July 2020. Statistical comparison was performed on the 9 samples from June/July 2020 only, to avoid non-independence due to repeated sampling of 3 donors. Supplementary Figure 4 ...
References
Briefing, SARS-CoV-2 variants of concern and variants under investigation in England
Cele, Jackson, Khan, SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection, doi:10.1101/2021.12.08.21267417
Choi, Koch, Wu, Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis, Nat Med
Corbett, Nason, Flach, Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates, Science
Elfström, Blomqvist, Nilsson, Differences in risk for SARS-CoV-2 infection among healthcare workers, Prev Med Rep
Freed, Silander, SARS-CoV2 genome sequencing protocol (1200bp amplicon 'midnight' primer set, using Nanopore Rapid kit)
Goldberg, Mandel, Bar-On, Waning Immunity after the BNT162b2 Vaccine in Israel, N Engl J Med
Greaney, Loes, Crawford, Comprehensive mapping of mutations in the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human plasma antibodies, Cell Host Microbe
Hansen, Baum, Pascal, Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science
Jones, Brown-Augsburger, Corbett, The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates, Sci Transl Med, doi:10.1126/scitranslmed.abf1906
Khoury, Cromer, Reynaldi, Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection, Nat Med
Klevebro, Bahram, Elfström, Risk of SARS-CoV-2 exposure among hospital healthcare workers in relation to patient contact and type of care, Scand J Public Health
Kuhlmann, Mayer, Claassen, Breakthrough Infections with SARS-CoV-2 Omicron Variant Despite Booster Dose of mRNA Vaccine, doi:10.2139/ssrn.3981711
Levin, Lustig, Cohen, Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months, N Engl J Med
Muecksch, Weisblum, Barnes, Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations, Immunity
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature
Seow, Graham, Merrick, Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans, Nat Microbiol
Sheward, Mandolesi, Urgard, Kim, Beta RBD boost broadens antibody-mediated protection against SARS-CoV-2 variants in animal models, Cell Rep
Wang, Muecksch, Schaefer-Babajew, Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection, Nature
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med
Wu, Li, Wang, Tolerability, Safety, Pharmacokinetics, and Immunogenicity of a Novel SARS-CoV-2 Neutralizing Antibody, Etesevimab, in Chinese Healthy Adults: a Randomized, Double-Blind, Placebo-Controlled, First-in-Human Phase 1 Study, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1101/2021.12.19.473354",
"URL": "http://dx.doi.org/10.1101/2021.12.19.473354",
"abstract": "<jats:p>The recently-emerged SARS-CoV-2 B.1.1.529 variant (Omicron) is spreading rapidly in many countries, with a spike that is highly diverged from the pandemic founder, raising fears that it may evade neutralizing antibody responses. We cloned the Omicron spike from a diagnostic sample which allowed us to rapidly establish an Omicron pseudotyped virus neutralization assay, sharing initial neutralization results only 13 days after the variant was first reported to the WHO, 8 days after receiving the sample. Here we show that Omicron is substantially resistant to neutralization by several monoclonal antibodies that form part of clinical cocktails. Further, we find neutralizing antibody responses in pooled reference sera sampled shortly after infection or vaccination are substantially less potent against Omicron, with neutralizing antibody titers reduced by up to 45 fold compared to those for the pandemic founder. Similarly, in a cohort of convalescent sera prior to vaccination, neutralization of Omicron was low to undetectable. However, in recent samples from two cohorts from Stockholm, Sweden, antibody responses capable of cross-neutralizing Omicron were prevalent. Sera from infected-then-vaccinated healthcare workers exhibited robust cross-neutralization of Omicron, with an average potency reduction of only 5-fold relative to the pandemic founder variant, and some donors showing no loss at all. A similar pattern was observed in randomly sampled recent blood donors, with an average 7-fold loss of potency. Both cohorts showed substantial between-donor heterogeneity in their ability to neutralize Omicron. Together, these data highlight the extensive but incomplete evasion of neutralizing antibody responses by the Omicron variant, and suggest that increasing the magnitude of neutralizing antibody responses by boosting with unmodified vaccines may suffice to raise titers to levels that are protective.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
12,
20
]
]
},
"author": [
{
"affiliation": [],
"family": "Sheward",
"given": "Daniel J.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kim",
"given": "Changil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ehling",
"given": "Roy A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pankow",
"given": "Alec",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9005-6774",
"affiliation": [],
"authenticated-orcid": false,
"family": "Castro Dopico",
"given": "Xaquin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin",
"given": "Darren P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reddy",
"given": "Sai T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dillner",
"given": "Joakim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karlsson Hedestam",
"given": "Gunilla B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albert",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murrell",
"given": "Ben",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
21
]
],
"date-time": "2021-12-21T03:00:12Z",
"timestamp": 1640055612000
},
"deposited": {
"date-parts": [
[
2021,
12,
21
]
],
"date-time": "2021-12-21T03:00:12Z",
"timestamp": 1640055612000
},
"group-title": "Immunology",
"indexed": {
"date-parts": [
[
2021,
12,
21
]
],
"date-time": "2021-12-21T06:51:11Z",
"timestamp": 1640069471560
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
12,
20
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.12.19.473354",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
12,
20
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
12,
20
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron)"
],
"type": "posted-content"
}
sheward
