Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial
et al., Heliyon, doi:10.1016/j.heliyon.2023.e22839, PROSPERO CRD42023412835, Nov 2023
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
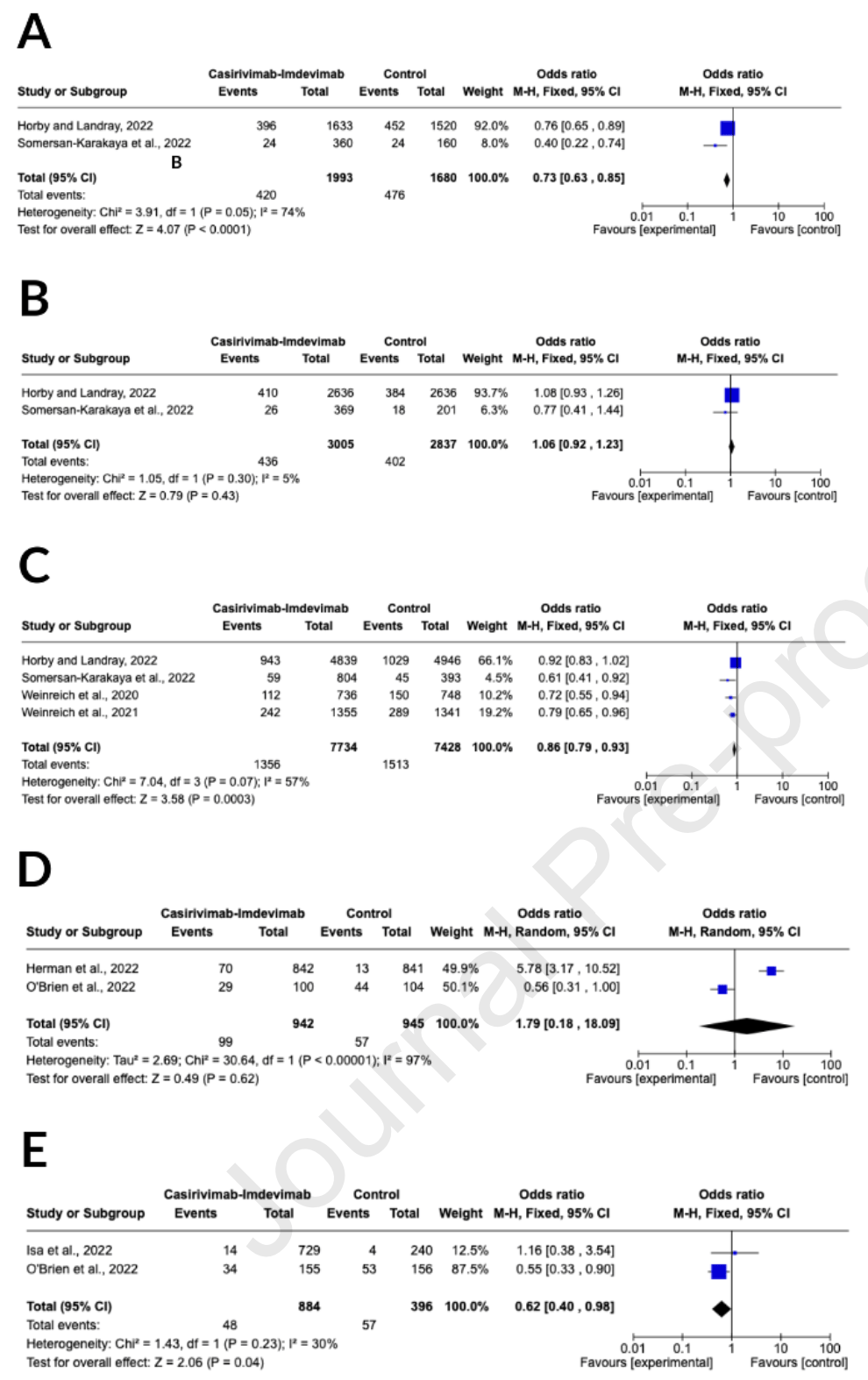
Systematic review and meta analysis showing lower mortality and progression, and improved viral load with casirivimab/imdevimab treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Currently there are 34 casirivimab/imdevimab studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 19% lower [-18‑45%] |
| Ventilation | 0% higher [-11‑13%] |
| ICU admission | 35% lower [-8‑60%] |
| Hospitalization | 39% lower [16‑55%] |
| Cases | 72% fewer [32‑89%] |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Wicaksono et al., 27 Nov 2023, peer-reviewed, 4 authors, trial PROSPERO CRD42023412835.
Contact: imam.adi@unpad.ac.id.
Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial
Heliyon, doi:10.1016/j.heliyon.2023.e22839
Background: The advantages and disadvantages of casirivimab-imdevimab for coronavirus disease 2019 are not well understood. We conducted a systematic review and meta-analysis of relevant literature to determine the therapeutic effectiveness and potential side effects of casirivimab-imdevimab in COVID-19 patients. Methods: Databases were searched from the time of their commencement until February 28 th , 2023. The primary results evaluated were the death rate at 28 days, progression of current clinical symptoms within 28 days, viral load, discharge from hospital, and any adverse events. Also, we contrasted the effects of the casirivimab-imdevimab treatment with placebo or standard of care. The protocol registration for this systematic review and meta-analysis was recorded in the PROSPERO database (CRD42023412835). Results: A total of eight studies were included, comprising 19,819 patients, and conducted a qualitative assessment of their risk of bias using the Cochrane risk of bias tool. Casirivimabimdevimab effectively reduced the mortality rate (OR = 0.62; 95% CI of 0.40-0.98; p = 0.04; I 2 = 30%) and reduced the progression of clinical symptoms (OR = 0.86; 95% CI of 0.79-0.93; p = 0.0003; I 2 = 57%). Casirivimab-imdevimab also improved viral load clearance and hospital discharge. Additionally, the trials' findings demonstrated a slight decrease in the likelihood of adverse events occurring with the use of casirivimab-imdevimab.
Conclusion: Our research suggests that casirivimab-imdevimab may be a valuable, safe, and effective anti-SARS-CoV-2 regimen.
Consent for publication Not applicable.
Competing interests All authors declare no conflict of interest.
Ethics approval and consent to participate Ethical approval was not needed because this is a meta-analysis.
Authors' contribution IAW and CS: conceptualization; CS: methodology and software; IAW, KME, and NW: validation; IAW, CS, and NW: formal analysis; KME and NW: investigation; CS: writingoriginal draft preparation; IAW, KME, and NW: review and editing; and IAW: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Declaration of interests ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J o u r n a l P r e -p r o o f
References
Abani, Abbas, Abbas, Abbas, Abbasi et al., Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, The Lancet, doi:10.1016/S0140-6736(22)00163-5
Angamo, Mohammed, Peterson, Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis, Infection, doi:10.1007/s15010-021-01671-0
Benaicha, Khenhrani, Veer, Devi, Shahbaz et al., Efficacy of Molnupiravir for the Treatment of Mild or Moderate COVID-19 in Adults: A Meta-Analysis, Cureus, doi:10.7759/cureus.38586
Bhattacharya, Chatterjee, Sharma, Lee, Chakraborty, Delta variant (B.1.617.2) of SARS-CoV-2: current understanding of infection, transmission, immune escape, and mutational landscape, Folia Microbiol (Praha), doi:10.1007/s12223-022-01001-3
Chen, Liang, Wu, Li, Wang et al., Advances and challenges in using nirmatrelvir and its derivatives against SARS-CoV-2 infection, J Pharm Anal, doi:10.1016/j.jpha.2022.10.005
Deeks, Casirivimab/Imdevimab: First Approval, Drugs, doi:10.1007/s40265-021-01620-z
Deng, Heybati, Ramaraju, Zhou, Rayner et al., Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis, Infection, doi:10.1007/s15010-022-01825-8
Fan, Zhang, Ma, Zhang, Safety profile of the antiviral drug remdesivir: An update, Biomedicine and Pharmacotherapy, doi:10.1016/j.biopha.2020.110532
Ghazanfar, Haider, Gurjar, Hernandez, Jyala et al., Outcomes of Monoclonal Antibody Infusion Treatment During Delta (B.1.617.2and Omicron (B.1.1.529) COVID 19 Variant Surges among Vaccinated and Unvaccinated Patients, Health Serv Insights, doi:10.1177/11786329221127153.JournalPre-proof
Haddad, Dokmak, Karaman, A Comprehensive Review on the Efficacy of Several Pharmacologic Agents for the Treatment of COVID-19, Life, doi:10.3390/life12111758
Hansen, Baum, Pascal, Russo, Giordano et al., Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science, doi:10.1126/science.abd0827
Herman, O'brien, Forleo-Neto, Sarkar, Isa et al., Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00416-9
Hernandez, Piscoya, Pasupuleti, Phan, Julakanti et al., Beneficial and Harmful Effects of Monoclonal Antibodies for the Treatment and Prophylaxis of COVID-19: Systematic Review and Meta-Analysis, American Journal of Medicine, doi:10.1016/j.amjmed.2022.06.019
Higgins, Altman, Gøtzsche, Jüni, Moher et al., The Cochrane Collaboration's tool for assessing J o u r n a l P r e -p r o o f risk of bias in randomised trials, BMJ (Online), doi:10.1136/bmj.d5928
Higgins, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0
Higgins, Thompson, Quantifying heterogeneity in a meta-analysis, Stat Med, doi:10.1002/sim.1186
Huang, Mccreary, Bariola, Minnier, Wadas et al., Effectiveness of Casirivimab-Imdevimab and Sotrovimab During a SARS-CoV-2 Delta Variant Surge, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20957
Isa, Forleo-Neto, Meyer, Zheng, Rasmussen et al., Repeat subcutaneous administration of casirivimab and imdevimab in adults is well-tolerated and prevents the occurrence of J o u r n a l P r e -p r o o f COVID-19, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.06.045
Jovanoski, Kuznik, Becker, Hussein, Briggs, Cost-effectiveness of casirivimab/imdevimab in patients with COVID-19 in the ambulatory setting, J Manag Care Spec Pharm, doi:10.18553/JMCP.2022.21469
Kim, Yoo, Bae, Kim, Lee, Effectiveness of Paxlovid, an Oral Antiviral Drug, Against the Omicron BA.5 Variant in Korea: Severe Progression and Death Between July and November 2022, J Korean Med Sci, doi:10.3346/jkms.2023.38.e211
Kokic, Hillen, Tegunov, Dienemann, Seitz et al., Mechanism of SARS-CoV-2 polymerase stalling by remdesivir, Nat Commun, doi:10.1038/s41467-020-20542-0
Lin, Hung, Lai, Wang, Chen, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials, J Med Virol, doi:10.1002/jmv.27623
Miranda, Mckinzie, Dobrovolsky, Revollo, Evaluation of the mutagenic effects of Molnupiravir and N4-hydroxycytidine in bacterial and mammalian cells by HiFi sequencing, Environ Mol Mutagen, doi:10.1002/em.22510.JournalPre-proof
Moher, Shamseer, Clarke, Ghersi, Liberati et al., Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement, Revista Espanola de Nutricion Humana y Dietetica, doi:10.1186/2046-4053-4-1
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clinical Infectious Diseases, doi:10.1093/cid/ciac443
O'brien, Forleo-Neto, Sarkar, Isa, Hou et al., Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.24939
Panda, Singh, Moirangthem, Bahurupi, Saha et al., Antiviral combination clinically better than standard therapy in severe but not in non-severe covid-19, Clin Pharmacol, doi:10.2147/CPAA.S325083.JournalPre-proof
Pontolillo, Ucciferri, Borrelli, Di Nicola, Vecchiet et al., Molnupiravir as an Early Treatment for COVID-19: A Real Life Study, Pathogens, doi:10.3390/pathogens11101121
Popping, Nichols, Appelman, Biemond, Vergouwe et al., Health Outcomes and Costeffectiveness of Monoclonal SARS-CoV-2 Antibodies as Pre-exposure Prophylaxis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.21985.JournalPre-proofWeinreich
Portal-Celhay, Forleo-Neto, Eagan, Musser, Davis et al., Virologic Efficacy of Casirivimab and Imdevimab COVID-19 Antibody Combination in Outpatients with SARS-CoV-2 Infection: A Phase 2 Dose-Ranging Randomized Clinical Trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.25411
Quiros-Roldan, Amadasi, Zanella, Antoni, Storti et al., Monoclonal antibodies against sars-cov-2: Current scenario and future perspectives, Pharmaceuticals, doi:10.3390/ph14121272
Razonable, Pawlowski, O'horo, Arndt, Arndt et al., Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101102
Ruggeri, Signorini, Caravaggio, Casirivimab and imdevimab: Costeffectiveness analysis of the treatment based on monoclonal antibodies on outpatients with Covid-19, PLoS One, doi:10.1371/journal.pone.0279022
Singh, De, Antiviral agents for the treatment of COVID-19: Progress and challenges, Cell Rep Med, doi:10.1016/j.xcrm.2022.100549
Somersan-Karakaya, Mylonakis, Menon, Wells, Ali et al., Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19, J Infect Dis, doi:10.1093/infdis/jiac320
Suhandi, Mohammed, Wilar, El-Rayyes, Wathoni, Effectiveness of Mesenchymal Stem Cell Secretome on Wound Healing: A Systematic Review and Meta-analysis, Tissue Eng Regen Med, doi:10.1007/s13770-023-00570-9
Tian, Feng, Chen, Efficacy and Safety of Molnupiravir Treatment for COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2023.106870
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, New England Journal of Medicine, doi:10.1056/nejmoa2035002
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., e -p r o o f REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, New England Journal of Medicine, doi:10.1056/nejmoa2108163
Zhuang, Xu, Wu, Yang, Lin et al., Postmarketing safety concerns with nirmatrelvir: A disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System, Br J Clin Pharmacol, doi:10.1111/bcp.15783
DOI record:
{
"DOI": "10.1016/j.heliyon.2023.e22839",
"ISSN": [
"2405-8440"
],
"URL": "http://dx.doi.org/10.1016/j.heliyon.2023.e22839",
"alternative-id": [
"S2405844023100478"
],
"article-number": "e22839",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Heliyon"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.heliyon.2023.e22839"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3793-5988",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wicaksono",
"given": "Imam Adi",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7746-3271",
"affiliation": [],
"authenticated-orcid": false,
"family": "Suhandi",
"given": "Cecep",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9555-1814",
"affiliation": [],
"authenticated-orcid": false,
"family": "Elamin",
"given": "Khaled M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5985-6909",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wathoni",
"given": "Nasrul",
"sequence": "additional"
}
],
"container-title": "Heliyon",
"container-title-short": "Heliyon",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T18:11:59Z",
"timestamp": 1701108719000
},
"deposited": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T18:12:21Z",
"timestamp": 1701108741000
},
"indexed": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T00:50:54Z",
"timestamp": 1701132654658
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 20,
"start": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T00:00:00Z",
"timestamp": 1700524800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844023100478?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844023100478?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e22839",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Antiviral combination clinically better than standard therapy in severe but not in non-severe covid-19",
"author": "Panda",
"journal-title": "Clin. Pharmacol.",
"key": "10.1016/j.heliyon.2023.e22839_bib1",
"volume": "13",
"year": "2021"
},
{
"article-title": "Antiviral agents for the treatment of COVID-19: progress and challenges",
"author": "Singh",
"journal-title": "Cell Rep Med",
"key": "10.1016/j.heliyon.2023.e22839_bib2",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/j.ijantimicag.2023.106870",
"article-title": "Efficacy and safety of molnupiravir treatment for COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Tian",
"doi-asserted-by": "crossref",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "10.1016/j.heliyon.2023.e22839_bib3",
"volume": "62",
"year": "2023"
},
{
"DOI": "10.7759/cureus.38586",
"article-title": "Efficacy of molnupiravir for the treatment of mild or moderate COVID-19 in adults: a meta-analysis",
"author": "Benaicha",
"doi-asserted-by": "crossref",
"journal-title": "Cureus",
"key": "10.1016/j.heliyon.2023.e22839_bib4",
"year": "2023"
},
{
"DOI": "10.1038/s41467-020-20542-0",
"article-title": "Mechanism of SARS-CoV-2 polymerase stalling by remdesivir",
"author": "Kokic",
"doi-asserted-by": "crossref",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.heliyon.2023.e22839_bib5",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s15010-021-01671-0",
"article-title": "Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis",
"author": "Angamo",
"doi-asserted-by": "crossref",
"journal-title": "Infection",
"key": "10.1016/j.heliyon.2023.e22839_bib6",
"volume": "50",
"year": "2022"
},
{
"article-title": "Effectiveness of Paxlovid, an oral antiviral drug, against the omicron BA.5 variant in Korea: severe progression and death between July and November 2022",
"author": "Kim",
"journal-title": "J. Kor. Med. Sci.",
"key": "10.1016/j.heliyon.2023.e22839_bib7",
"volume": "38",
"year": "2023"
},
{
"author": "Najjar-Debbiny",
"key": "10.1016/j.heliyon.2023.e22839_bib8",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1111/bcp.15783",
"article-title": "Post-marketing safety concerns with nirmatrelvir: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System",
"author": "Zhuang",
"doi-asserted-by": "crossref",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "10.1016/j.heliyon.2023.e22839_bib9",
"volume": "89",
"year": "2023"
},
{
"DOI": "10.1016/j.jpha.2022.10.005",
"article-title": "Advances and challenges in using nirmatrelvir and its derivatives against SARS-CoV-2 infection",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "J Pharm Anal",
"key": "10.1016/j.heliyon.2023.e22839_bib10",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1002/em.22510",
"article-title": "Evaluation of the mutagenic effects of Molnupiravir and N4-hydroxycytidine in bacterial and mammalian cells by HiFi sequencing",
"author": "Miranda",
"doi-asserted-by": "crossref",
"journal-title": "Environ. Mol. Mutagen.",
"key": "10.1016/j.heliyon.2023.e22839_bib11",
"volume": "63",
"year": "2022"
},
{
"DOI": "10.3390/pathogens11101121",
"article-title": "Molnupiravir as an early treatment for COVID-19: a real life study",
"author": "Pontolillo",
"doi-asserted-by": "crossref",
"journal-title": "Pathogens",
"key": "10.1016/j.heliyon.2023.e22839_bib12",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2020.110532",
"article-title": "Safety profile of the antiviral drug remdesivir: an update",
"author": "Fan",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.heliyon.2023.e22839_bib13",
"volume": "130",
"year": "2020"
},
{
"article-title": "Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail",
"author": "Hansen",
"first-page": "369",
"journal-title": "Science",
"key": "10.1016/j.heliyon.2023.e22839_bib14",
"volume": "1979",
"year": "2020"
},
{
"DOI": "10.3390/life12111758",
"article-title": "A comprehensive review on the efficacy of several pharmacologic agents for the treatment of COVID-19",
"author": "Haddad",
"doi-asserted-by": "crossref",
"journal-title": "Life",
"key": "10.1016/j.heliyon.2023.e22839_bib15",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101102",
"article-title": "Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19",
"author": "Razonable",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.heliyon.2023.e22839_bib16",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.heliyon.2023.e22839_bib17",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.d5928",
"article-title": "The Cochrane Collaboration's tool for assessing risk of bias in randomised trials",
"author": "Higgins",
"doi-asserted-by": "crossref",
"journal-title": "Br. Med. J.",
"key": "10.1016/j.heliyon.2023.e22839_bib18",
"volume": "343",
"year": "2011"
},
{
"DOI": "10.1007/s13770-023-00570-9",
"article-title": "Effectiveness of mesenchymal stem cell secretome on wound healing: a systematic review and meta-analysis",
"author": "Suhandi",
"doi-asserted-by": "crossref",
"journal-title": "Tissue Eng Regen Med",
"key": "10.1016/j.heliyon.2023.e22839_bib19",
"year": "2023"
},
{
"author": "Moher",
"key": "10.1016/j.heliyon.2023.e22839_bib20",
"volume": "20",
"year": "2016"
},
{
"author": "G.S",
"key": "10.1016/j.heliyon.2023.e22839_bib21",
"series-title": "Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0",
"year": "2011"
},
{
"DOI": "10.1002/sim.1186",
"article-title": "Quantifying heterogeneity in a meta-analysis",
"author": "Higgins",
"doi-asserted-by": "crossref",
"journal-title": "Stat. Med.",
"key": "10.1016/j.heliyon.2023.e22839_bib22",
"volume": "21",
"year": "2002"
},
{
"DOI": "10.1016/S1473-3099(22)00416-9",
"article-title": "Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial",
"author": "Herman",
"doi-asserted-by": "crossref",
"first-page": "1444",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.heliyon.2023.e22839_bib23",
"volume": "22",
"year": "2022"
},
{
"article-title": "Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Abani",
"journal-title": "Lancet",
"key": "10.1016/j.heliyon.2023.e22839_bib24",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2022.06.045",
"article-title": "Repeat subcutaneous administration of casirivimab and imdevimab in adults is well-tolerated and prevents the occurrence of COVID-19",
"author": "Isa",
"doi-asserted-by": "crossref",
"journal-title": "Int. J. Infect. Dis.",
"key": "10.1016/j.heliyon.2023.e22839_bib25",
"volume": "122",
"year": "2022"
},
{
"article-title": "Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial",
"author": "O'Brien",
"journal-title": "JAMA",
"key": "10.1016/j.heliyon.2023.e22839_bib26",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.25411",
"article-title": "Virologic efficacy of casirivimab and imdevimab COVID-19 antibody combination in outpatients with SARS-CoV-2 infection: a phase 2 dose-ranging randomized clinical trial",
"author": "Portal-Celhay",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.heliyon.2023.e22839_bib27",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac320",
"article-title": "Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19",
"author": "Somersan-Karakaya",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.heliyon.2023.e22839_bib28",
"volume": "227",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.heliyon.2023.e22839_bib29",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.amjmed.2022.06.019",
"article-title": "Beneficial and harmful effects of monoclonal antibodies for the treatment and prophylaxis of COVID-19: systematic review and meta-analysis",
"author": "Hernandez",
"doi-asserted-by": "crossref",
"journal-title": "Am. J. Med.",
"key": "10.1016/j.heliyon.2023.e22839_bib30",
"volume": "135",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27623",
"article-title": "The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: a systematic review and meta-analysis of randomized controlled trials",
"author": "Lin",
"doi-asserted-by": "crossref",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.heliyon.2023.e22839_bib31",
"volume": "94",
"year": "2022"
},
{
"article-title": "An eua for casirivimab and imdevimab for COVID-19",
"first-page": "62",
"journal-title": "Med. Lett. Drugs Ther.",
"key": "10.1016/j.heliyon.2023.e22839_bib32",
"year": "2020"
},
{
"DOI": "10.1007/s15010-022-01825-8",
"article-title": "Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Infection",
"key": "10.1016/j.heliyon.2023.e22839_bib33",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.3390/ph14121272",
"article-title": "Monoclonal antibodies against sars-cov-2: current scenario and future perspectives",
"author": "Quiros-Roldan",
"doi-asserted-by": "crossref",
"journal-title": "Pharmaceuticals",
"key": "10.1016/j.heliyon.2023.e22839_bib34",
"volume": "14",
"year": "2021"
},
{
"author": "Food and Drug Administration",
"key": "10.1016/j.heliyon.2023.e22839_bib35",
"series-title": "Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of REGEN-COV (Casirivimab and Imdevimab)",
"year": "2023"
},
{
"author": "Deeks",
"key": "10.1016/j.heliyon.2023.e22839_bib36",
"series-title": "Casirivimab/Imdevimab: First Approval",
"year": "2021"
},
{
"DOI": "10.1007/s12223-022-01001-3",
"article-title": "Delta variant (B.1.617.2) of SARS-CoV-2: current understanding of infection, transmission, immune escape, and mutational landscape",
"author": "Bhattacharya",
"doi-asserted-by": "crossref",
"journal-title": "Folia Microbiol.",
"key": "10.1016/j.heliyon.2023.e22839_bib37",
"volume": "68",
"year": "2023"
},
{
"article-title": "Outcomes of monoclonal antibody infusion treatment during delta (B.1.617.2) and omicron (B.1.1.529) COVID 19 variant surges among vaccinated and unvaccinated patients",
"author": "Ghazanfar",
"journal-title": "Health Serv. Insights",
"key": "10.1016/j.heliyon.2023.e22839_bib38",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20957",
"article-title": "Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 delta variant surge",
"author": "Huang",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.heliyon.2023.e22839_bib39",
"volume": "5",
"year": "2022"
},
{
"article-title": "Cost-effectiveness of casirivimab/imdevimab in patients with COVID-19 in the ambulatory setting",
"author": "Jovanoski",
"journal-title": "J Manag Care Spec Pharm",
"key": "10.1016/j.heliyon.2023.e22839_bib40",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0279022",
"article-title": "Casirivimab and imdevimab: cost-effectiveness analysis of the treatment based on monoclonal antibodies on outpatients with Covid-19",
"author": "Ruggeri",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.heliyon.2023.e22839_bib41",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2023.21985",
"article-title": "Health outcomes and cost-effectiveness of monoclonal SARS-CoV-2 antibodies as pre-exposure prophylaxis",
"author": "Popping",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.heliyon.2023.e22839_bib42",
"volume": "6",
"year": "2023"
}
],
"reference-count": 42,
"references-count": 42,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2405844023100478"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Efficacy and safety of casirivimab-imdevimab combination on COVID-19 patients: A systematic review and meta-analysis randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
