A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data
et al., European Urology, doi:10.1016/j.eururo.2021.12.013, NCT04475601, Dec 2021
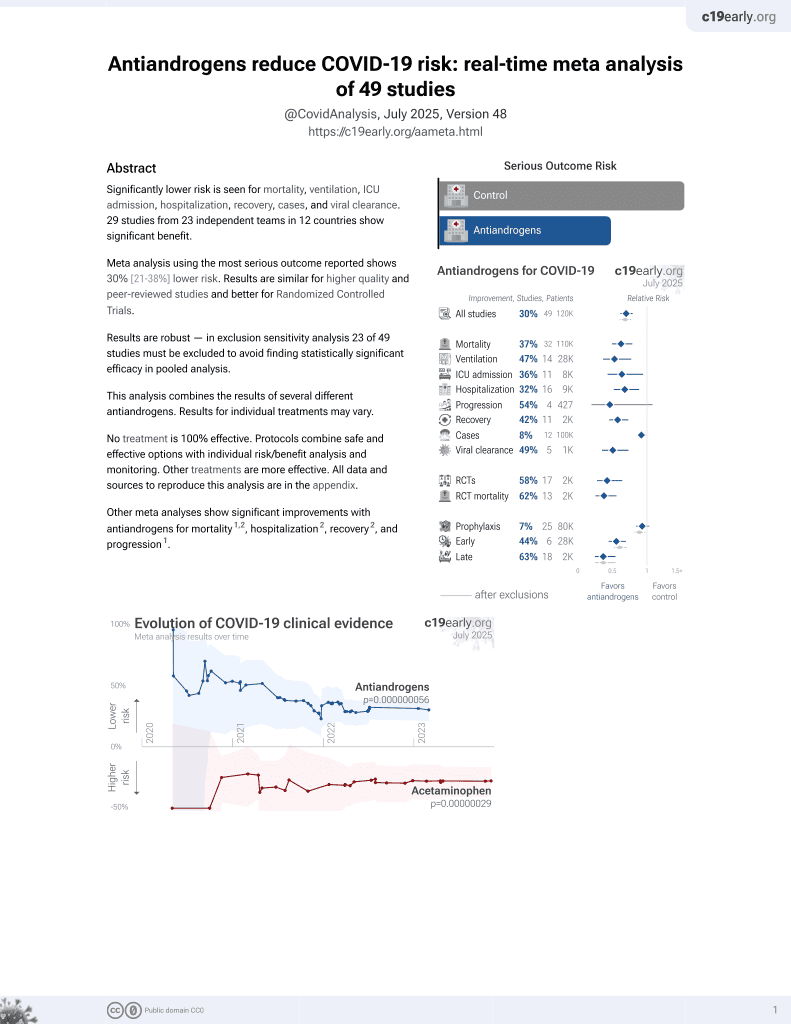
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 7,894 COVID+ prostate cancer patients, analyzing patients on antiandrogen treatment, ADT, and ADT + abiraterone acetate or enzalutamide, showing mixed results and higher mortality for ADT + abiraterone acetate or enzalutamide. This paper also includes a small RCT which is listed separately, and an in vitro HBEC study showing no significant differences (p = 0.084). The supplementary data is not currently available.
|
risk of death, 2.0% lower, HR 0.98, p = 0.94, treatment 21 of 358 (5.9%), control 167 of 4,980 (3.4%), adjusted per study, antiandrogen treatment.
|
|
risk of death, 11.0% lower, HR 0.89, p = 0.66, treatment 20 of 334 (6.0%), control 167 of 4,980 (3.4%), adjusted per study, ADT.
|
|
risk of death, 151.0% higher, HR 2.51, p < 0.001, treatment 24 of 152 (15.8%), control 167 of 4,980 (3.4%), adjusted per study, ADT and abiraterone acetate or enzalutamide.
|
|
risk of ICU admission, 28.0% higher, HR 1.28, p = 0.28, treatment 24 of 358 (6.7%), control 216 of 4,980 (4.3%), adjusted per study, antiandrogen treatment.
|
|
risk of ICU admission, 13.0% lower, HR 0.87, p = 0.62, treatment 16 of 334 (4.8%), control 216 of 4,980 (4.3%), adjusted per study, ADT.
|
|
risk of ICU admission, 21.0% lower, HR 0.79, p = 0.60, treatment 6 of 152 (3.9%), control 216 of 4,980 (4.3%), adjusted per study, ADT and abiraterone acetate or enzalutamide.
|
|
risk of hospitalization, 23.0% higher, HR 1.23, p = 0.09, treatment 126 of 358 (35.2%), control 1,108 of 4,980 (22.2%), adjusted per study, antiandrogen treatment.
|
|
risk of hospitalization, 24.0% higher, HR 1.24, p = 0.09, treatment 126 of 334 (37.7%), control 1,108 of 4,980 (22.2%), adjusted per study, ADT.
|
|
risk of hospitalization, 40.0% higher, HR 1.40, p = 0.06, treatment 66 of 152 (43.4%), control 1,108 of 4,980 (22.2%), adjusted per study, ADT and abiraterone acetate or enzalutamide.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Welén et al., 14 Dec 2021, retrospective, Sweden, peer-reviewed, 27 authors, trial NCT04475601 (history).
Contact: andreas.josefsson@umu.se.
DOI record:
{
"DOI": "10.1016/j.eururo.2021.12.013",
"ISSN": [
"0302-2838"
],
"URL": "http://dx.doi.org/10.1016/j.eururo.2021.12.013",
"alternative-id": [
"S0302283821022247"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "European Urology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eururo.2021.12.013"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier B.V. on behalf of European Association of Urology."
}
],
"author": [
{
"affiliation": [],
"family": "Welén",
"given": "Karin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rosendal",
"given": "Ebba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gisslén",
"given": "Magnus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lenman",
"given": "Annasara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freyhult",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fonseca-Rodríguez",
"given": "Osvaldo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bremell",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stranne",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balkhed",
"given": "Åse Östholm",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Niward",
"given": "Katarina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Repo",
"given": "Johanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinsson",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henningsson",
"given": "Anna J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Styrke",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Angelin",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindquist",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allard",
"given": "Annika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Becker",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rudolfsson",
"given": "Stina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buckland",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carlsson",
"given": "Camilla Thellenberg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bjartell",
"given": "Anders",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nilsson",
"given": "Anna C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahlm",
"given": "Clas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Connolly",
"given": "Anne-Marie Fors",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Överby",
"given": "Anna K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Josefsson",
"given": "Andreas",
"sequence": "additional"
}
],
"container-title": "European Urology",
"container-title-short": "European Urology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T22:42:54Z",
"timestamp": 1639608174000
},
"deposited": {
"date-parts": [
[
2022,
2,
26
]
],
"date-time": "2022-02-26T03:38:20Z",
"timestamp": 1645846700000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T10:40:13Z",
"timestamp": 1711622413583
},
"is-referenced-by-count": 38,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T00:00:00Z",
"timestamp": 1639526400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0302283821022247?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0302283821022247?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "285-293",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41467-020-19741-6",
"article-title": "Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission",
"author": "Peckham",
"doi-asserted-by": "crossref",
"first-page": "6317",
"journal-title": "Nat Commun",
"key": "10.1016/j.eururo.2021.12.013_b0005",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "Factors associated with COVID-19-related death using OpenSAFELY",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Nature",
"key": "10.1016/j.eururo.2021.12.013_b0010",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.12.091082",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eururo.2021.12.013_b0015",
"unstructured": "Ghazizadeh Z, Majd H, Richter M, et al. Androgen regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. BioRxiv preprint. https://doi.org/10.1101/2020.05.12.091082"
},
{
"DOI": "10.1038/s41467-021-24342-y",
"article-title": "The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells",
"author": "Leach",
"doi-asserted-by": "crossref",
"first-page": "4068",
"journal-title": "Nat Commun",
"key": "10.1016/j.eururo.2021.12.013_b0020",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.mce.2009.12.022",
"article-title": "Androgen receptor and androgen-dependent gene expression in lung",
"author": "Mikkonen",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Mol Cell Endocrinol",
"key": "10.1016/j.eururo.2021.12.013_b0025",
"volume": "317",
"year": "2010"
},
{
"article-title": "Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats",
"author": "Dalpiaz",
"journal-title": "PLoS One",
"key": "10.1016/j.eururo.2021.12.013_b0030",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532)",
"author": "Montopoli",
"doi-asserted-by": "crossref",
"first-page": "1040",
"journal-title": "Ann Oncol",
"key": "10.1016/j.eururo.2021.12.013_b0035",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"article-title": "Does androgen deprivation therapy protect against severe complications from COVID-19?",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Ann Oncol",
"key": "10.1016/j.eururo.2021.12.013_b0040",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.05.079",
"article-title": "Androgenetic alopecia present in the majority of hospitalized COVID-19 patients: the “Gabrin sign”",
"author": "Wambier",
"doi-asserted-by": "crossref",
"first-page": "680",
"journal-title": "J Am Acad Dermatol",
"key": "10.1016/j.eururo.2021.12.013_b0045",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.015",
"article-title": "Androgen deprivation and SARS-CoV-2 in men with prostate cancer",
"author": "Koskinen",
"doi-asserted-by": "crossref",
"first-page": "1417",
"journal-title": "Ann Oncol",
"key": "10.1016/j.eururo.2021.12.013_b0050",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/0895-4356(94)90129-5",
"article-title": "Validation of a combined comorbidity index",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/j.eururo.2021.12.013_b0055",
"volume": "47",
"year": "1994"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"article-title": "A new method of classifying prognostic comorbidity in longitudinal studies: development and validation",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "J Chronic Dis",
"key": "10.1016/j.eururo.2021.12.013_b0060",
"volume": "40",
"year": "1987"
},
{
"DOI": "10.2147/CLEP.S282475",
"article-title": "Adaptation of the Charlson comorbidity index for register-based research in Sweden",
"author": "Ludvigsson",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Clin Epidemiol",
"key": "10.1016/j.eururo.2021.12.013_b0065",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s40262-015-0283-1",
"article-title": "Pharmacokinetic drug interaction studies with enzalutamide",
"author": "Gibbons",
"doi-asserted-by": "crossref",
"first-page": "1057",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/j.eururo.2021.12.013_b0070",
"volume": "54",
"year": "2015"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eururo.2021.12.013_b0075",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1097/JU.0000000000002180",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eururo.2021.12.013_b0080",
"unstructured": "Lyon M, Li J, Cullen J, et al. 5α-Reductase inhibitors are associated with reduced risk of SARS-CoV-2 infection: a matched-pair, registry-based analysis. J Urol. In press. https://doi.org/10.1097/ju.0000000000002180."
},
{
"article-title": "Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial",
"author": "Cadegiani",
"journal-title": "Cureus",
"key": "10.1016/j.eururo.2021.12.013_b0085",
"volume": "13",
"year": "2021"
},
{
"article-title": "Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2",
"author": "Qiao",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.eururo.2021.12.013_b0090",
"volume": "118",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21171-x",
"article-title": "Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "866",
"journal-title": "Nat Commun",
"key": "10.1016/j.eururo.2021.12.013_b0095",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.668698",
"article-title": "Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial",
"author": "McCoy",
"doi-asserted-by": "crossref",
"journal-title": "Front Med",
"key": "10.1016/j.eururo.2021.12.013_b0100",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2355-0",
"article-title": "Viral and host factors related to the clinical outcome of COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "437",
"journal-title": "Nature",
"key": "10.1016/j.eururo.2021.12.013_b0105",
"volume": "583",
"year": "2020"
},
{
"key": "10.1016/j.eururo.2021.12.013_b0110",
"unstructured": "Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J. In press. https://doi.org/10.22037/uj.v18i.6691."
},
{
"DOI": "10.1158/1078-0432.CCR-16-2339",
"article-title": "A phase I/Ib Study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer",
"author": "Schwartzberg",
"doi-asserted-by": "crossref",
"first-page": "4046",
"journal-title": "Clin Cancer Res",
"key": "10.1016/j.eururo.2021.12.013_b0115",
"volume": "23",
"year": "2017"
},
{
"DOI": "10.1093/cid/ciaa644",
"article-title": "Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "2428",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eururo.2021.12.013_b0120",
"volume": "71",
"year": "2020"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0302283821022247"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Urology"
],
"subtitle": [],
"title": "A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "81"
}