Astegolimab or Efmarodocokin Alfa in Patients With Severe COVID-19 Pneumonia: A Randomized, Phase 2 Trial
et al., Critical Care Medicine, doi:10.1097/CCM.0000000000005716, COVASTIL, NCT04386616, Nov 2022
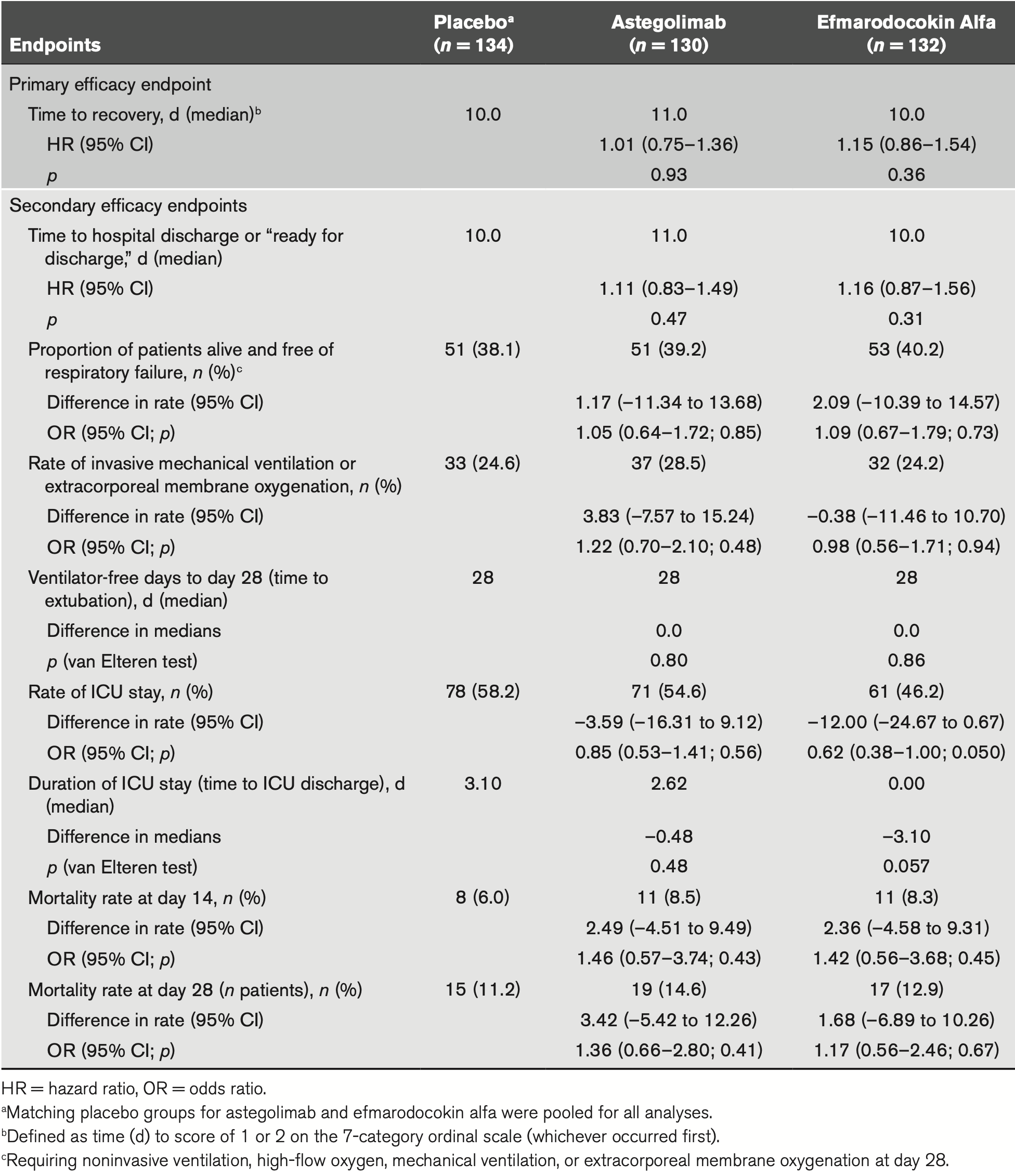
RCT 396 hospitalized patients with severe COVID-19 pneumonia showing no significant difference in time to recovery with astegolimab (IL-33 receptor blocker) or efmarodocokin alfa (IL-22 pathway activator). Median time to recovery was 11 days for astegolimab, 10 days for efmarodocokin alfa, and 10 days for placebo (p=0.93 and p=0.36 respectively). Neither drug showed benefit for secondary endpoints including mortality, hospital discharge time, ICU admission, or mechanical ventilation.
Study covers astegolimab and efmarodocokin alfa.
|
risk of death, 14.8% higher, RR 1.15, p = 0.67, treatment 17 of 132 (12.9%), control 15 of 134 (11.2%), odds ratio converted to relative risk.
|
|
risk of no recovery, 13.0% lower, HR 0.87, p = 0.35, treatment 132, control 134, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Waters et al., 14 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, median age 57.0, 20 authors, study period June 2020 - March 2021, average treatment delay 11.58 days, trial NCT04386616 (history) (COVASTIL).
Astegolimab or Efmarodocokin Alfa in Patients With Severe COVID-19 Pneumonia: A Randomized, Phase 2 Trial*
Critical Care Medicine, doi:10.1097/ccm.0000000000005716
OBJECTIVES: Severe cases of COVID-19 pneumonia can lead to acute respiratory distress syndrome (ARDS). Release of interleukin (IL)-33, an epithelial-derived alarmin, and IL-33/ST2 pathway activation are linked with ARDS development in other viral infections. IL-22, a cytokine that modulates innate immunity through multiple regenerative and protective mechanisms in lung epithelial cells, is reduced in patients with ARDS. This study aimed to evaluate safety and efficacy of astegolimab, a human immunoglobulin G2 monoclonal antibody that selectively inhibits the IL-33 receptor, ST2, or efmarodocokin alfa, a human IL-22 fusion protein that activates IL-22 signaling, for treatment of severe COVID-19 pneumonia.
DESIGN: Phase 2, double-blind, placebo-controlled study (COVID-astegolimab-IL).
SETTING: Hospitals. PATIENTS: Hospitalized adults with severe COVID-19 pneumonia.
INTERVENTIONS: Patients were randomized to receive IV astegolimab, efmarodocokin alfa, or placebo, plus standard of care. The primary endpoint was time to recovery, defined as time to a score of 1 or 2 on a 7-category ordinal scale by day 28.
MEASUREMENTS AND MAIN RESULTS: The study randomized 396 patients. Median time to recovery was 11 days (hazard ratio [HR], 1.01 d; p = 0.93) and 10 days (HR, 1.15 d; p = 0.38) for astegolimab and efmarodocokin alfa, respectively, versus 10 days for placebo. Key secondary endpoints (improved recovery, mortality, or prevention of worsening) showed no treatment benefits. No new safety signals were observed and adverse events were similar across treatment arms. Biomarkers demonstrated that both drugs were pharmacologically active.
CONCLUSIONS: Treatment with astegolimab or efmarodocokin alfa did not improve time to recovery in patients with severe COVID-19 pneumonia.
pathway modulation for treatment of hospitalized patients with COVID-19 pneumonia. Although both astegolimab and efmarodocokin alfa induced pharmacodynamic activity and were safe and well tolerated in this patient population, neither drug showed a significant difference from placebo in time to recovery, the primary endpoint, or in any of the secondary endpoints. The AEs, SAEs, and fatal events were consistent
References
Abood, Mchugh, Rich, IL-22-binding protein exacerbates influenza, bacterial super-infection, Mucosal Immunol
Ali, Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19, J Med Virol
Angriman, Ferreyro, Burry, Interleukin-6 receptor blockade in patients with COVID-19: Placing clinical trials into context, Lancet Respir Med
Bajwa, Volk, Christiani, Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome, Crit Care Med
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Burke, Freeman, Cellura, Inflammatory phenotyping predicts clinical outcome in COVID-19, Respir Res
Cevikbas, Steinhoff, IL-33: A novel danger signal system in atopic dermatitis, J Invest Dermatol
Choi, Mcaleer, Zheng, Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia, J Exp Med
Core, R: A Language and Environment for Statistical Computing
Garg, Patel, Pham, Clinical trends among U.S. adults hospitalized with COVID-19, March to December 2020: A cross-sectional study, Ann Intern Med
Goletti, Cantini, Baricitinib therapy in Covid-19 pneumonia -an unmet need fulfilled, N Engl J Med
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Herold, Jurinovic, Arnreich, Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J Allergy Clin Immunol
Hoegl, Bachmann, Scheiermann, Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury, Am J Respir Cell Mol Biol
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Ito, Hirose, Saku, IL-22 induces Reg3γ and inhibits allergic inflammation in house dust mite-induced asthma models, J Exp Med
Ivanov, Renneson, Fontaine, Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection, J Virol
Kelsen, Agache, Soong, Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial, J Allergy Clin Immunol
Lee, Zhong, Pai, Nonclinical safety assessment of a human interleukin-22FC IG fusion protein demonstrates in vitro to in vivo and cross-species translatability, Pharmacol Res Perspect
Lin, Fu, Wang, Inflammation elevated IL-33 originating from the lung mediates inflammation in acute lung injury, Clin Immunol
Luo, Zhou, Yan, Prognostic value of C-reactive protein in patients with coronavirus 2019, Clin Infect Dis
Pociask, Scheller, Mandalapu, IL-22 is essential for lung epithelial repair following influenza infection, Am J Pathol
Rosas, Brau, Waters, Tocilizumab in hospitalized patients with severe Covid-19 pneumonia, N Engl J Med
Rothenberg, Wang, Lekkerkerker, Randomized phase I healthy volunteer study of UTTR1147A (IL-22Fc): A potential therapy for epithelial injury, Clin Pharmacol Ther
Stanczak, Sanin, Apostolova, IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals, Nat Commun
Staton, Peng, Owen, A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers, BMC Pulm Med
Stefanich, Sukumaran, Pre-clinical and translational pharmacology of a human interleukin-22 IgG fusion protein for potential treatment of infectious or inflammatory diseases, Biochem Pharmacol
Sánchez-Marteles, Rubio-Gracia, Pena-Fresneda, Early measurement of blood sST2 is a good predictor of death and poor outcomes in patients admitted for COVID-19 infection, J Clin Med
Tzotzos, Fischer, Fischer, Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey, Crit Care
Whittington, Armstrong, Uppington, Interleukin-22: A potential immunomodulatory molecule in the lung, Am J Respir Cell Mol Biol
Wu, Hu, Cai, Interleukin 22 attenuated angiotensin II induced acute lung injury through inhibiting the apoptosis of pulmonary microvascular endothelial cells, Sci Rep
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Xue, Zhao, Ying, IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway, Antiviral Res
Yeates, Nahmias, Chinn, Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure, PLoS One
Zhang, Xu, Zhao, Diagnostic value of sST2 in cardiovascular diseases: A systematic review and meta-analysis, Front Cardiovasc Med
Zheng, Li, Tian, HIP/PAP protects against bleomycin-induced lung injury and inflammation and subsequent fibrosis in mice, J Cell Mol Med
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
Zizzo, Cohen, Imperfect storm: Is interleukin-33 the Achilles heel of COVID-19?, Lancet Rheumatol
DOI record:
{
"DOI": "10.1097/ccm.0000000000005716",
"ISSN": [
"0090-3493"
],
"URL": "http://dx.doi.org/10.1097/CCM.0000000000005716",
"abstract": "<jats:sec>\n <jats:title>OBJECTIVES:</jats:title>\n <jats:p>Severe cases of COVID-19 pneumonia can lead to acute respiratory distress syndrome (ARDS). Release of interleukin (IL)-33, an epithelial-derived alarmin, and IL-33/ST2 pathway activation are linked with ARDS development in other viral infections. IL-22, a cytokine that modulates innate immunity through multiple regenerative and protective mechanisms in lung epithelial cells, is reduced in patients with ARDS. This study aimed to evaluate safety and efficacy of astegolimab, a human immunoglobulin G2 monoclonal antibody that selectively inhibits the IL-33 receptor, ST2, or efmarodocokin alfa, a human IL-22 fusion protein that activates IL-22 signaling, for treatment of severe COVID-19 pneumonia.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>DESIGN:</jats:title>\n <jats:p>Phase 2, double-blind, placebo-controlled study (COVID-astegolimab-IL).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>SETTING:</jats:title>\n <jats:p>Hospitals.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>PATIENTS:</jats:title>\n <jats:p>Hospitalized adults with severe COVID-19 pneumonia.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>INTERVENTIONS:</jats:title>\n <jats:p>Patients were randomized to receive IV astegolimab, efmarodocokin alfa, or placebo, plus standard of care. The primary endpoint was time to recovery, defined as time to a score of 1 or 2 on a 7-category ordinal scale by day 28.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>MEASUREMENTS AND MAIN RESULTS:</jats:title>\n <jats:p>The study randomized 396 patients. Median time to recovery was 11 days (hazard ratio [HR], 1.01 d; <jats:italic toggle=\"yes\">p</jats:italic> = 0.93) and 10 days (HR, 1.15 d; <jats:italic toggle=\"yes\">p</jats:italic> = 0.38) for astegolimab and efmarodocokin alfa, respectively, versus 10 days for placebo. Key secondary endpoints (improved recovery, mortality, or prevention of worsening) showed no treatment benefits. No new safety signals were observed and adverse events were similar across treatment arms. Biomarkers demonstrated that both drugs were pharmacologically active.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>CONCLUSIONS:</jats:title>\n <jats:p>Treatment with astegolimab or efmarodocokin alfa did not improve time to recovery in patients with severe COVID-19 pneumonia.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Velocity Clinical Research, Chula Vista, CA."
}
],
"family": "Waters",
"given": "Michael",
"sequence": "first"
},
{
"affiliation": [
{
"name": "David Geffen School of Medicine, Harbor-UCLA Medical Center, Torrance, CA."
},
{
"name": "Milefchik-Rand Medical Group, Torrance Memorial Medical Center, Torrance, CA."
}
],
"family": "McKinnell",
"given": "James A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE."
}
],
"family": "Kalil",
"given": "Andre C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, Emory University School of Medicine, and Grady Health System, Atlanta, GA."
}
],
"family": "Martin",
"given": "Greg S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, Emory University School of Medicine, Atlanta, GA."
}
],
"family": "Buchman",
"given": "Timothy G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Early Clinical Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Theess",
"given": "Wiebke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Product Development Data Sciences, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Yang",
"given": "Xiaoying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biomarker Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Lekkerkerker",
"given": "Annemarie N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biomarker Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Staton",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biomarker Discovery, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Rosenberger",
"given": "Carrie M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Immunology Discovery, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Pappu",
"given": "Rajita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Wang",
"given": "Yehong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Zhang",
"given": "Wenhui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Brooks",
"given": "Logan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Early Clinical Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Cheung",
"given": "Dorothy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Product Development Safety, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Galanter",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Early Clinical Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Chen",
"given": "Hubert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Early Clinical Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Mohan",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Early Clinical Development, Genentech, Inc., South San Francisco, CA."
}
],
"family": "Peck",
"given": "Melicent C.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the COVID-astegolimab-interleukin (IL) (COVASTIL) Study Group",
"sequence": "additional"
}
],
"container-title": "Critical Care Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"lww.com",
"ovid.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
15
]
],
"date-time": "2022-12-15T15:44:53Z",
"timestamp": 1671119093000
},
"deposited": {
"date-parts": [
[
2025,
5,
2
]
],
"date-time": "2025-05-02T17:01:15Z",
"timestamp": 1746205275000
},
"indexed": {
"date-parts": [
[
2025,
10,
24
]
],
"date-time": "2025-10-24T16:48:12Z",
"timestamp": 1761324492190,
"version": "3.40.4"
},
"is-referenced-by-count": 17,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
11,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
14
]
],
"date-time": "2022-11-14T00:00:00Z",
"timestamp": 1668384000000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.1097/CCM.0000000000005716",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "103-116",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2022,
11,
14
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
14
]
]
},
"published-print": {
"date-parts": [
[
2023,
1
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China.",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "R1-20250502",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "R2-20250502",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention.",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "R3-20250502",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study.",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "R4-20250502",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03240-7",
"article-title": "Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey.",
"author": "Tzotzos",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Crit Care",
"key": "R5-20250502",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1038/jid.2012.66",
"article-title": "IL-33: A novel danger signal system in atopic dermatitis.",
"author": "Cevikbas",
"doi-asserted-by": "crossref",
"first-page": "1326",
"journal-title": "J Invest Dermatol",
"key": "R6-20250502",
"volume": "132",
"year": "2012"
},
{
"article-title": "Inflammation elevated IL-33 originating from the lung mediates inflammation in acute lung injury.",
"author": "Lin",
"first-page": "30535",
"journal-title": "Clin Immunol",
"key": "R7-20250502",
"volume": "S1521-6616",
"year": "2016"
},
{
"DOI": "10.1016/S2665-9913(20)30340-4",
"article-title": "Imperfect storm: Is interleukin-33 the Achilles heel of COVID-19?",
"author": "Zizzo",
"doi-asserted-by": "crossref",
"first-page": "e779",
"journal-title": "Lancet Rheumatol",
"key": "R8-20250502",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-22449-w",
"article-title": "IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals.",
"author": "Stanczak",
"doi-asserted-by": "crossref",
"first-page": "2133",
"journal-title": "Nat Commun",
"key": "R9-20250502",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/s12931-020-01511-z",
"article-title": "Inflammatory phenotyping predicts clinical outcome in COVID-19.",
"author": "Burke",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "Respir Res",
"key": "R10-20250502",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2021.03.044",
"article-title": "Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial.",
"author": "Kelsen",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "J Allergy Clin Immunol",
"key": "R11-20250502",
"volume": "148",
"year": "2021"
},
{
"DOI": "10.1165/rcmb.2009-0440OC",
"article-title": "Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury.",
"author": "Hoegl",
"doi-asserted-by": "crossref",
"first-page": "369",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "R12-20250502",
"volume": "44",
"year": "2011"
},
{
"DOI": "10.1038/s41598-017-02056-w",
"article-title": "Interleukin 22 attenuated angiotensin II induced acute lung injury through inhibiting the apoptosis of pulmonary microvascular endothelial cells.",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "2210",
"journal-title": "Sci Rep",
"key": "R13-20250502",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1165/rcmb.2003-0285OC",
"article-title": "Interleukin-22: A potential immunomodulatory molecule in the lung.",
"author": "Whittington",
"doi-asserted-by": "crossref",
"first-page": "220",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "R14-20250502",
"volume": "31",
"year": "2004"
},
{
"DOI": "10.1016/j.ajpath.2012.12.007",
"article-title": "IL-22 is essential for lung epithelial repair following influenza infection.",
"author": "Pociask",
"doi-asserted-by": "crossref",
"first-page": "1286",
"journal-title": "Am J Pathol",
"key": "R15-20250502",
"volume": "182",
"year": "2013"
},
{
"DOI": "10.1016/j.bcp.2018.03.031",
"article-title": "Pre-clinical and translational pharmacology of a human interleukin-22 IgG fusion protein for potential treatment of infectious or inflammatory diseases.",
"author": "Stefanich",
"doi-asserted-by": "crossref",
"first-page": "224",
"journal-title": "Biochem Pharmacol",
"key": "R16-20250502",
"volume": "152",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2028700",
"article-title": "Tocilizumab in hospitalized patients with severe Covid-19 pneumonia.",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1503",
"journal-title": "N Engl J Med",
"key": "R17-20250502",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 - final report.",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "R18-20250502",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa641",
"article-title": "Prognostic value of C-reactive protein in patients with coronavirus 2019.",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "2174",
"journal-title": "Clin Infect Dis",
"key": "R20-20250502",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26097",
"article-title": "Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19.",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "2409",
"journal-title": "J Med Virol",
"key": "R21-20250502",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1002/prp2.434",
"article-title": "Nonclinical safety assessment of a human interleukin-22FC IG fusion protein demonstrates in vitro to in vivo and cross-species translatability.",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e00434",
"journal-title": "Pharmacol Res Perspect",
"key": "R22-20250502",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.1002/cpt.1164",
"article-title": "Randomized phase I healthy volunteer study of UTTR1147A (IL-22Fc): A potential therapy for epithelial injury.",
"author": "Rothenberg",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Clin Pharmacol Ther",
"key": "R23-20250502",
"volume": "105",
"year": "2019"
},
{
"DOI": "10.3390/jcm10163534",
"article-title": "Early measurement of blood sST2 is a good predictor of death and poor outcomes in patients admitted for COVID-19 infection.",
"author": "Sánchez-Marteles",
"doi-asserted-by": "crossref",
"first-page": "3534",
"journal-title": "J Clin Med",
"key": "R24-20250502",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1186/s12890-018-0763-9",
"article-title": "A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers.",
"author": "Staton",
"doi-asserted-by": "crossref",
"first-page": "5",
"journal-title": "BMC Pulm Med",
"key": "R25-20250502",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.3389/fcvm.2021.697837",
"article-title": "Diagnostic value of sST2 in cardiovascular diseases: A systematic review and meta-analysis.",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "697837",
"journal-title": "Front Cardiovasc Med",
"key": "R26-20250502",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0b013e3182978f91",
"article-title": "Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome.",
"author": "Bajwa",
"doi-asserted-by": "crossref",
"first-page": "2521",
"journal-title": "Crit Care Med",
"key": "R27-20250502",
"volume": "41",
"year": "2013"
},
{
"DOI": "10.1111/jcmm.15334",
"article-title": "HIP/PAP protects against bleomycin-induced lung injury and inflammation and subsequent fibrosis in mice.",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "6804",
"journal-title": "J Cell Mol Med",
"key": "R28-20250502",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1084/jem.20162108",
"article-title": "IL-22 induces Reg3γ and inhibits allergic inflammation in house dust mite-induced asthma models.",
"author": "Ito",
"doi-asserted-by": "crossref",
"first-page": "3037",
"journal-title": "J Exp Med",
"key": "R29-20250502",
"volume": "214",
"year": "2017"
},
{
"DOI": "10.1128/JVI.02943-12",
"article-title": "Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection.",
"author": "Ivanov",
"doi-asserted-by": "crossref",
"first-page": "6911",
"journal-title": "J Virol",
"key": "R30-20250502",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1084/jem.20120260",
"article-title": "Innate Stat3-mediated induction of the antimicrobial protein Reg3γ is required for host defense against MRSA pneumonia.",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "551",
"journal-title": "J Exp Med",
"key": "R31-20250502",
"volume": "210",
"year": "2013"
},
{
"DOI": "10.1016/j.antiviral.2017.03.006",
"article-title": "IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway.",
"author": "Xue",
"doi-asserted-by": "crossref",
"first-page": "68",
"journal-title": "Antiviral Res",
"key": "R32-20250502",
"volume": "142",
"year": "2017"
},
{
"DOI": "10.1038/s41385-019-0188-7",
"article-title": "IL-22-binding protein exacerbates influenza, bacterial super-infection.",
"author": "Abood",
"doi-asserted-by": "crossref",
"first-page": "1231",
"journal-title": "Mucosal Immunol",
"key": "R33-20250502",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1016/j.jaci.2020.05.008",
"article-title": "Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19.",
"author": "Herold",
"doi-asserted-by": "crossref",
"first-page": "128",
"journal-title": "J Allergy Clin Immunol",
"key": "R34-20250502",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.7326/M21-1991",
"article-title": "Clinical trends among U.S. adults hospitalized with COVID-19, March to December 2020: A cross-sectional study.",
"author": "Garg",
"doi-asserted-by": "crossref",
"first-page": "1409",
"journal-title": "Ann Intern Med",
"key": "R35-20250502",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0253767",
"article-title": "Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure.",
"author": "Yeates",
"doi-asserted-by": "crossref",
"first-page": "e0253767",
"journal-title": "PLoS One",
"key": "R36-20250502",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00139-9",
"article-title": "Interleukin-6 receptor blockade in patients with COVID-19: Placing clinical trials into context.",
"author": "Angriman",
"doi-asserted-by": "crossref",
"first-page": "655",
"journal-title": "Lancet Respir Med",
"key": "R37-20250502",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMe2034982",
"article-title": "Baricitinib therapy in Covid-19 pneumonia - an unmet need fulfilled.",
"author": "Goletti",
"doi-asserted-by": "crossref",
"first-page": "867",
"journal-title": "N Engl J Med",
"key": "R38-20250502",
"volume": "384",
"year": "2021"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/CCM.0000000000005716"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Astegolimab or Efmarodocokin Alfa in Patients With Severe COVID-19 Pneumonia: A Randomized, Phase 2 Trial*",
"type": "journal-article",
"update-policy": "https://doi.org/10.1097/lww.0000000000001000",
"volume": "51"
}
waters2