Recombinant C1 inhibitor in the prevention of severe COVID-19: a randomized, open-label, multi-center phase IIa trial
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1255292, PROTECT-COVID-19, NCT04414631, Oct 2023
RCT 84 hospitalized COVID-19 patients showing higher mortality with conestat alfa (recombinant C1 inhibitor), without reaching statistical significance.
|
risk of death, 237.5% higher, RR 3.38, p = 0.26, treatment 7 of 56 (12.5%), control 1 of 27 (3.7%), extended SAE followup.
|
|
risk of death, 889.3% higher, RR 9.89, p = 0.17, treatment 6 of 56 (10.7%), control 0 of 27 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28.
|
|
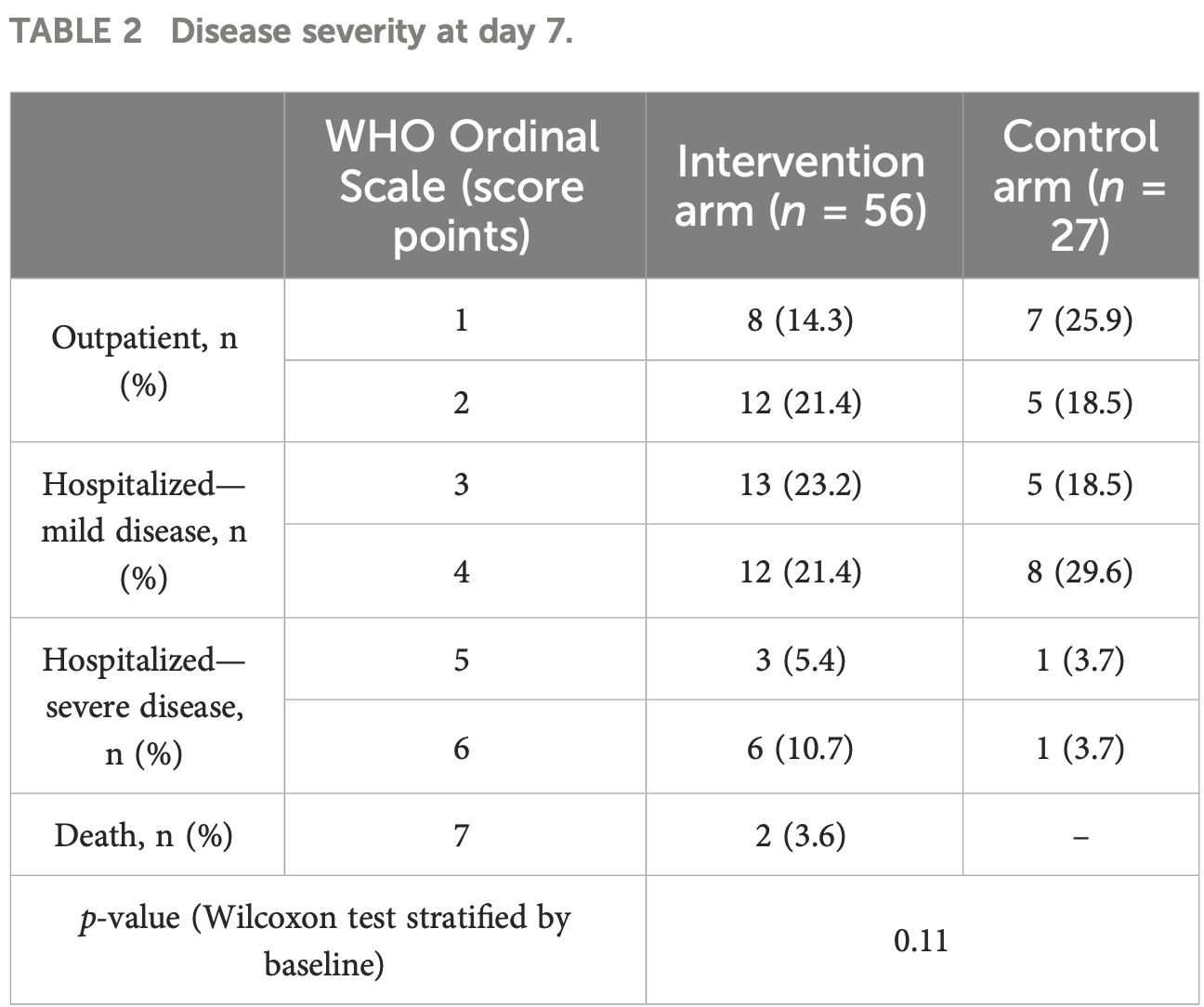
risk of death, 296.4% higher, RR 3.96, p = 1.00, treatment 2 of 56 (3.6%), control 0 of 27 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 7.
|
|
risk of mechanical ventilation, 285.7% higher, RR 3.86, p = 0.26, treatment 8 of 56 (14.3%), control 1 of 27 (3.7%), day 14.
|
|
risk of ICU admission, 141.1% higher, RR 2.41, p = 0.32, treatment 10 of 56 (17.9%), control 2 of 27 (7.4%), day 14.
|
|
risk of no hospital discharge, 117.0% higher, RR 2.17, p = 0.12, treatment 18 of 56 (32.1%), control 4 of 27 (14.8%), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Urwyler et al., 27 Oct 2023, Randomized Controlled Trial, multiple countries, peer-reviewed, mean age 61.1, 15 authors, study period August 2020 - March 2021, trial NCT04414631 (history) (PROTECT-COVID-19).
Contact: michael.osthoff@usb.ch.
Recombinant C1 inhibitor in the prevention of severe COVID-19: a randomized, open-label, multi-center phase IIa trial
Frontiers in Immunology, doi:10.3389/fimmu.2023.1255292
Background: Conestat alfa (ConA), a recombinant human C1 inhibitor, may prevent thromboinflammation. Methods: We conducted a randomized, open-label, multi-national clinical trial in which hospitalized adults at risk for progression to severe COVID-19 were assigned in a 2:1 ratio to receive either 3 days of ConA plus standard of care (SOC) or SOC alone. Primary and secondary endpoints were day 7 disease severity on the WHO Ordinal Scale, time to clinical improvement within 14 days, and safety, respectively.
Results: The trial was prematurely terminated because of futility after randomization of 84 patients, 56 in the ConA and 28 in the control arm. At baseline, higher WHO Ordinal Scale scores were more frequently observed in the ConA than in the control arm. On day 7, no relevant differences in the primary outcome were noted between the two arms (p = 0.11). The median time to defervescence was 3 days, and the median time to clinical improvement was 7 days in both arms (p = 0.22 and 0.56, respectively). Activation of plasma cascades and endothelial cells over time was similar in both groups. The incidence of adverse events (AEs) was higher in the intervention arm (any AE, 30% with ConA vs. 19% with SOC alone; serious AE, 27% vs. 15%; death, 11% vs. 0%). None of these were judged as being related to the study drug.
Ethics statement The studies involving humans were approved by the ethics committees «Ethikkommission Nordwest-und Zentralschweiz», «Ethikkommission Ostschweiz», and "Kantonale Ethikkommission Zürich» in Switzerland, "Comissaô Nacional de E ́tica Em Pesquisa" (CONEP) in Brazil and "Comisioń Federal Para La Proteccioń Contra Riesgos Sanitarios" (COFEPRIS) in Mexico. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conflict of interest MT reports receiving grants from the Swiss National Science Foundation, and having research collaborations with Roche, Novartis, and Idorsia outside the submitted work. WA reports receiving fees and research grants from A. Vogel AG, Gilead, and OM Pharma and fees for attendance of advisory boards to Pfizer, MSD Vifor Pharma, GSK, Sanofi, OM Pharma, and Janssen that were paid to his institution outside the submitted work. MO reports receiving grants from the Swiss National Science Foundation, consulting fees from Pharming Biotechnologies B.V. during the conduct of the study and grants from Pharming Biotechnologies B.V. outside the submitted work. LH reports receiving consulting fees from GlaxoSmithKline and Novartis during the conduct of the study but unrelated to this trial. The remaining authors declare that the research was conducted in the absence of any..
References
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19, N Engl J Med, doi:10.1056/NEJMoa2015432
Amara, Flierl, Rittirsch, Klos, Chen et al., Molecular intercommunication between the complement and coagulation systems, J Immunol, doi:10.4049/jimmunol.0903678
Annane, Pittock, Kulkarni, Pickering, Khoshnevis et al., Intravenous ravulizumab in mechanically ventilated patients hospitalised with severe COVID-19: a phase 3, multicentre, open-label, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(23)00082-6
Bekassy, Fagerstrom, Bader, Karpman, Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation, Nat Rev Immunol, doi:10.1038/s41577-021-00634-8
Boechat, Chora, Morais, Delgado, The immune response to SARS-CoV-2 and COVID-19 immunopathology -Current perspectives, Pulmonology, doi:10.1016/j.pulmoe.2021.03.008
Bork, Steffensen, Machnig, Treatment with C1-esterase inhibitor concentrate in type I or II hereditary angioedema: a systematic literature review, Allergy Asthma Proc, doi:10.2500/aap.2013.34.3677
Busch, Timmermans, Nagy, Visser, Huckriede et al., Neutrophils and contact activation of coagulation as potential drivers of COVID-19, Circulation, doi:10.1161/CIRCULATIONAHA.120.050656
Cai, Dole, Bergmeier, Scafidi, Feng et al., A direct role for C1 inhibitor in regulation of leukocyte adhesion, J Immunol, doi:10.4049/jimmunol.174.10.6462
Carragher, Robertson, Assessing safety at the end of clinical trials using system organ classes: A case and comparative study, Pharm Stat, doi:10.1002/pst.2148
Carvelli, Demaria, Vely, Batista, Benmansour et al., Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis, Nature, doi:10.1136/jitc-2020-SITC2020.0483
Castanha, Tuttle, Kitsios, Jacobs, Braga-Neto et al., IgG response to SARS-CoV-2 and seasonal coronaviruses contributes to complement overactivation in severe COVID-19 patients, J Infect Dis, doi:10.1093/infdis/jiac091
Charitos, Heijnen, Egli, Bassetti, Trendelenburg et al., Functional activity of the complement system in hospitalized COVID-19 patients: A prospective cohort study, Front Immunol, doi:10.3389/fimmu.2021.765330
Cicardi, Zingale, Zanichelli, Pappalardo, Cicardi, C1 inhibitor: molecular and clinical aspects, Springer Semin Immunopathol, doi:10.1007/s00281-005-0001-4
Conde, Cruz, Zhang, Lopez, Afshar-Kharghan, Platelet activation leads to activation and propagation of the complement system, J Exp Med, doi:10.1084/jem.20041497
Cuker, Tseng, Schünemann, Angchaisuksiri, Blair et al., American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis for patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients, Blood Adv, doi:10.1182/bloodadvances.2022007940
Davis, Bernstein, Conestat alfa for the treatment of angioedema attacks, Ther Clin Risk Manage, doi:10.2147/TCRM.S15544
De Nooijer, Grondman, Janssen, Netea, Willems et al., Complement activation in the disease course of COVID-19 and its effects on clinical outcomes, J Infect Dis, doi:10.1093/infdis/jiaa646
Gao, Hu, Zhang, Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation, Signal Transduct Target Ther, doi:10.1101/2020.03.29.20041962
Gesuete, Storini, Fantin, Stravalaci, Zanier et al., Recombinant C1 inhibitor in brain ischemic injury, Ann neurology, doi:10.1002/ana.21740
Holter, Pischke, De Boer, Lind, Jenum et al., Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients, Proc Natl Acad Sci, doi:10.1073/pnas.2010540117
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Kanse, Gallenmueller, Zeerleder, Stephan, Rannou et al., Factor VII-activating protease is activated in multiple trauma patients and generates anaphylatoxin C5a, J Immunol, doi:10.4049/jimmunol.1103029
Lipcsey, Persson, Eriksson, Blom, Fromell et al., The outcome of critically ill COVID-19 patients is linked to thromboinflammation dominated by the kallikrein/kinin system, Front Immunol, doi:10.3389/fimmu.2021.627579
Macor, Durigutto, Mangogna, Bussani, Maso et al., Multiple-organ complement deposition on vascular endothelium in COVID-19 patients, Biomedicines, doi:10.3390/biomedicines9081003
Magro, Mulvey, Berlin, Nuovo, Salvatore et al., Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases, Transl Res, doi:10.1016/j.trsl.2020.04.007
Malchair, Giol, Garcia, Rodriguez, Ruibal et al., Three-day icatibant on top of standard care in patients with COVID-19 pneumonia (ICAT.COVID): a randomized, open-label, phase 2, proof-of-concept trial, Clin Infect Dis, doi:10.1093/cid/ciac984
Mansour, Palma, Ulaf, Ribeiro, Bernardes et al., Safety and outcomes associated with the pharmacological inhibition of the kininkallikrein system in severe COVID-19, Viruses, doi:10.3390/v13020309
Narota, Puri, Singh, Kumar, Naura, COVID-19 and ARDS: Update on preventive and therapeutic venues, Curr Mol Med, doi:10.2174/1566524021666210408103921
Opal, Phylogenetic and functional relationships between coagulation and the innate immune response, Crit Care Med, doi:10.1097/00003246-200009001-00017
Pagano, Salmanton-Garcia, Marchesi, Busca, Corradini et al., COVID-19 infection in adult patients with hematological Malignancies: a European Hematology Association Survey (EPICOVIDEHA), J Hematol Oncol, doi:10.1186/s13045-021-01177-0
Peerschke, Valentino, So, Shulman, Ravinder, Thromboinflammation supports complement activation in cancer patients with COVID-19, Front Immunol, doi:10.3389/fimmu.2021.716361
Peerschke, Yin, Ghebrehiwet, Complement activation on platelets: implications for vascular inflammation and thrombosis, Mol Immunol, doi:10.1016/j.molimm.2010.05.009
Peoples, Strang, Complement activation in the central nervous system: A biophysical model for immune dysregulation in the disease state, Front Mol Neurosci, doi:10.3389/fnmol.2021.620090
Puy, Pang, Reitsma, Lorentz, Tucker et al., Cross-talk between the complement pathway and the contact activation system of coagulation: activated factor XI neutralizes complement factor H, J Immunol, doi:10.4049/jimmunol.2000398
Ravindran, Grys, Welch, Schapira, Patston, Inhibition of plasma kallikrein by C1-inhibitor: role of endothelial cells and the amino-terminal domain of C1-inhibitor, Thromb Haemost, doi:10.1160/TH04-01-0008
Rennét, Grüner, Schuh, Pauer, Burfeind, Defective thrombus formation in mice lacking coagulation factor XII, J Exp Med, doi:10.1084/jem.20050664
Ruggenenti, Marco, Cortinovis, Lorini, Sala et al., Eculizumab in patients with severe coronavirus disease 2019 (COVID-19) requiring continuous positive airway pressure ventilator support: Retrospective cohort study, PloS One, doi:10.1371/journal.pone.0261113
Sinkovits, Mezőb, Reti, Müller, Ivańyi et al., Complement overactivation and consumption predicts in-hospital mortality in SARS-coV-2 infection, Front Immunol, doi:10.3389/fimmu.2021.663187
Stravalaci, Pagani, Paraboschi, Pedotti, Doni et al., Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules, Nat Immunol, doi:10.1038/s41590-021-01114-w
Thomas, Dzieciatkowska, Hill, Francis, Hudson, Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level, J Proteome Res, doi:10.1021/acs.jproteome.0c00365
Urwyler, Charitos, Moser, Heijnen, Trendelenburg et al., Recombinant human C1 esterase inhibitor (conestat alfa) in the prevention of severe SARS-CoV-2 infection in hospitalized patients with COVID-19: A structured summary of a study protocol for a randomized, parallel-group, open-label, multi-center pilot trial (PROTECT-COVID-19), Trials, doi:10.1186/s13063-020-04976-x
Urwyler, Moser, Charitos, Heijnen, Rudin et al., Treatment of COVID-19 with conestat alfa, a regulator of the complement, contact activation and kallikrein-kinin system, Front Immunol, doi:10.3389/fimmu.2020.02072
Van Veen, Koiter, Vogelezang, Van Wessel, Van Dam et al., Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits, J Biotechnol, doi:10.1016/j.jbiotec.2012.09.005
Vlaar, Witzenrath, Van Paassen, Heunks, Mourvillier et al., Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00297-1
Wu, Huang, Sun, Xie, Lei et al., Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19, mSphere, doi:10.1128/mSphere.00362-20
Yu, Yuan, Chen, Chaturvedi, Braunstein et al., Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition, Blood, doi:10.1182/blood.2020008248
DOI record:
{
"DOI": "10.3389/fimmu.2023.1255292",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2023.1255292",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Conestat alfa (ConA), a recombinant human C1 inhibitor, may prevent thromboinflammation.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We conducted a randomized, open-label, multi-national clinical trial in which hospitalized adults at risk for progression to severe COVID-19 were assigned in a 2:1 ratio to receive either 3 days of ConA plus standard of care (SOC) or SOC alone. Primary and secondary endpoints were day 7 disease severity on the WHO Ordinal Scale, time to clinical improvement within 14 days, and safety, respectively.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The trial was prematurely terminated because of futility after randomization of 84 patients, 56 in the ConA and 28 in the control arm. At baseline, higher WHO Ordinal Scale scores were more frequently observed in the ConA than in the control arm. On day 7, no relevant differences in the primary outcome were noted between the two arms (<jats:italic>p</jats:italic> = 0.11). The median time to defervescence was 3 days, and the median time to clinical improvement was 7 days in both arms (<jats:italic>p</jats:italic> = 0.22 and 0.56, respectively). Activation of plasma cascades and endothelial cells over time was similar in both groups. The incidence of adverse events (AEs) was higher in the intervention arm (any AE, 30% with ConA vs. 19% with SOC alone; serious AE, 27% vs. 15%; death, 11% vs. 0%). None of these were judged as being related to the study drug.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The study results do not support the use of ConA to prevent COVID-19 progression.</jats:p></jats:sec><jats:sec><jats:title>Clinical trial registration</jats:title><jats:p><jats:uri>https://clinicaltrials.gov</jats:uri>, identifier NCT04414631.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fimmu.2023.1255292"
],
"article-number": "1255292",
"author": [
{
"affiliation": [],
"family": "Urwyler",
"given": "Pascal",
"sequence": "first"
},
{
"affiliation": [],
"family": "Leimbacher",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charitos",
"given": "Panteleimon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moser",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heijnen",
"given": "Ingmar A. F. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trendelenburg",
"given": "Marten",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thoma",
"given": "Reto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sumer",
"given": "Johannes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camacho-Ortiz",
"given": "Adrián",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bacci",
"given": "Marcelo R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huber",
"given": "Lars C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stüssi-Helbling",
"given": "Melina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albrich",
"given": "Werner C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sendi",
"given": "Parham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osthoff",
"given": "Michael",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
10,
28
]
],
"date-time": "2023-10-28T21:06:21Z",
"timestamp": 1698527181000
},
"deposited": {
"date-parts": [
[
2023,
10,
28
]
],
"date-time": "2023-10-28T21:06:29Z",
"timestamp": 1698527189000
},
"funder": [
{
"DOI": "10.13039/501100001711",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001711",
"id-type": "DOI"
}
],
"name": "Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung"
}
],
"indexed": {
"date-parts": [
[
2025,
11,
6
]
],
"date-time": "2025-11-06T20:16:08Z",
"timestamp": 1762460168985,
"version": "3.41.2"
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2023,
10,
27
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
27
]
],
"date-time": "2023-10-27T00:00:00Z",
"timestamp": 1698364800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1255292/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
10,
27
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
27
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.2174/1566524021666210408103921",
"article-title": "COVID-19 and ARDS: Update on preventive and therapeutic venues",
"author": "Narota",
"doi-asserted-by": "publisher",
"journal-title": "Curr Mol Med",
"key": "B1",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.pulmoe.2021.03.008",
"article-title": "The immune response to SARS-CoV-2 and COVID-19 immunopathology - Current perspectives",
"author": "Boechat",
"doi-asserted-by": "publisher",
"journal-title": "Pulmonology.",
"key": "B2",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1097/00003246-200009001-00017",
"article-title": "Phylogenetic and functional relationships between coagulation and the innate immune response",
"author": "Opal",
"doi-asserted-by": "publisher",
"journal-title": "Crit Care Med",
"key": "B3",
"volume": "28",
"year": "2000"
},
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19",
"author": "Ackermann",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B4",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41577-021-00634-8",
"article-title": "Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation",
"author": "Bekassy",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B5",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2010540117",
"article-title": "Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients",
"author": "Holter",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci USA",
"key": "B6",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa646",
"article-title": "Complement activation in the disease course of COVID-19 and its effects on clinical outcomes",
"author": "de Nooijer",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis",
"key": "B7",
"year": "2020"
},
{
"DOI": "10.1136/jitc-2020-SITC2020.0483",
"article-title": "Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis",
"author": "Carvelli",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B8",
"year": "2020"
},
{
"DOI": "10.1021/acs.jproteome.0c00365",
"article-title": "Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level",
"author": "D'Alessandro",
"doi-asserted-by": "publisher",
"journal-title": "J Proteome Res",
"key": "B9",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1128/mSphere.00362-20",
"article-title": "Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "mSphere",
"key": "B10",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.663187",
"article-title": "Complement overactivation and consumption predicts in-hospital mortality in SARS-coV-2 infection",
"author": "Sinkovits",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B11",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s00281-005-0001-4",
"article-title": "C1 inhibitor: molecular and clinical aspects",
"author": "Cicardi",
"doi-asserted-by": "publisher",
"journal-title": "Springer Semin Immunopathol",
"key": "B12",
"volume": "27",
"year": "2005"
},
{
"DOI": "10.4049/jimmunol.174.10.6462",
"article-title": "A direct role for C1 inhibitor in regulation of leukocyte adhesion",
"author": "Cai",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B13",
"volume": "174",
"year": "2005"
},
{
"DOI": "10.1084/jem.20050664",
"article-title": "Defective thrombus formation in mice lacking coagulation factor XII",
"author": "Renné",
"doi-asserted-by": "publisher",
"journal-title": "J Exp Med",
"key": "B14",
"volume": "202",
"year": "2005"
},
{
"DOI": "10.2147/TCRM.S15544",
"article-title": "Conestat alfa for the treatment of angioedema attacks",
"author": "Davis",
"doi-asserted-by": "publisher",
"journal-title": "Ther Clin Risk Manage",
"key": "B15",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.1016/j.jbiotec.2012.09.005",
"article-title": "Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits",
"author": "van Veen",
"doi-asserted-by": "publisher",
"journal-title": "J Biotechnol",
"key": "B16",
"volume": "162",
"year": "2012"
},
{
"DOI": "10.1002/ana.21740",
"article-title": "Recombinant C1 inhibitor in brain ischemic injury",
"author": "Gesuete",
"doi-asserted-by": "publisher",
"journal-title": "Ann neurology",
"key": "B17",
"volume": "66",
"year": "2009"
},
{
"DOI": "10.3389/fimmu.2020.02072",
"article-title": "Treatment of COVID-19 with conestat alfa, a regulator of the complement, contact activation and kallikrein-kinin system",
"author": "Urwyler",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B18",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04976-x",
"article-title": "Recombinant human C1 esterase inhibitor (conestat alfa) in the prevention of severe SARS-CoV-2 infection in hospitalized patients with COVID-19: A structured summary of a study protocol for a randomized, parallel-group, open-label, multi-center pilot trial (PROTECT-COVID-19)",
"author": "Urwyler",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Trials.",
"key": "B19",
"volume": "22",
"year": "2021"
},
{
"key": "B20",
"volume-title": "IDSA Guidelines on the Treatment and Management of Patients with COVID-19 idsociety.org",
"year": "2022"
},
{
"DOI": "10.1182/bloodadvances.2022007940",
"article-title": "American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis for patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients",
"author": "Cuker",
"doi-asserted-by": "publisher",
"journal-title": "Blood Adv",
"key": "B21",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1002/pst.2148",
"article-title": "Assessing safety at the end of clinical trials using system organ classes: A case and comparative study",
"author": "Carragher",
"doi-asserted-by": "publisher",
"journal-title": "Pharm Stat",
"key": "B22",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "B23",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.765330",
"article-title": "Functional activity of the complement system in hospitalized COVID-19 patients: A prospective cohort study",
"author": "Charitos",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B24",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.050656",
"article-title": "Neutrophils and contact activation of coagulation as potential drivers of COVID-19",
"author": "Busch",
"doi-asserted-by": "publisher",
"journal-title": "Circulation.",
"key": "B25",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.3389/fnmol.2021.620090",
"article-title": "Complement activation in the central nervous system: A biophysical model for immune dysregulation in the disease state",
"author": "Peoples",
"doi-asserted-by": "publisher",
"journal-title": "Front Mol Neurosci",
"key": "B26",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1160/TH04-01-0008",
"article-title": "Inhibition of plasma kallikrein by C1-inhibitor: role of endothelial cells and the amino-terminal domain of C1-inhibitor",
"author": "Ravindran",
"doi-asserted-by": "publisher",
"journal-title": "Thromb Haemost",
"key": "B27",
"volume": "92",
"year": "2004"
},
{
"DOI": "10.1093/infdis/jiac091",
"article-title": "IgG response to SARS-CoV-2 and seasonal coronaviruses contributes to complement overactivation in severe COVID-19 patients",
"author": "Castanha",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis",
"key": "B28",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.627579",
"article-title": "The outcome of critically ill COVID-19 patients is linked to thromboinflammation dominated by the kallikrein/kinin system",
"author": "Lipcsey",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B29",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1101/2020.03.29.20041962",
"article-title": "Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "318",
"journal-title": "Signal Transduct Target Ther",
"key": "B30",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.trsl.2020.04.007",
"article-title": "Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases",
"author": "Magro",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Transl Res",
"key": "B31",
"volume": "220",
"year": "2020"
},
{
"DOI": "10.1038/s41590-021-01114-w",
"article-title": "Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules",
"author": "Stravalaci",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B32",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1182/blood.2020008248",
"article-title": "Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition",
"author": "Yu",
"doi-asserted-by": "publisher",
"journal-title": "Blood.",
"key": "B33",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.3390/biomedicines9081003",
"article-title": "Multiple-organ complement deposition on vascular endothelium in COVID-19 patients",
"author": "Macor",
"doi-asserted-by": "publisher",
"journal-title": "Biomedicines",
"key": "B34",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.2000398",
"article-title": "Cross-talk between the complement pathway and the contact activation system of coagulation: activated factor XI neutralizes complement factor H",
"author": "Puy",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B35",
"volume": "206",
"year": "2021"
},
{
"DOI": "10.1084/jem.20041497",
"article-title": "Platelet activation leads to activation and propagation of the complement system",
"author": "Del Conde",
"doi-asserted-by": "publisher",
"journal-title": "J Exp Med",
"key": "B36",
"volume": "201",
"year": "2005"
},
{
"DOI": "10.3389/fimmu.2021.716361",
"article-title": "Thromboinflammation supports complement activation in cancer patients with COVID-19",
"author": "Peerschke",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B37",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.molimm.2010.05.009",
"article-title": "Complement activation on platelets: implications for vascular inflammation and thrombosis",
"author": "Peerschke",
"doi-asserted-by": "publisher",
"journal-title": "Mol Immunol",
"key": "B38",
"volume": "47",
"year": "2010"
},
{
"DOI": "10.4049/jimmunol.0903678",
"article-title": "Molecular intercommunication between the complement and coagulation systems",
"author": "Amara",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B39",
"volume": "185",
"year": "2010"
},
{
"DOI": "10.4049/jimmunol.1103029",
"article-title": "Factor VII-activating protease is activated in multiple trauma patients and generates anaphylatoxin C5a",
"author": "Kanse",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B40",
"volume": "188",
"year": "2012"
},
{
"DOI": "10.1371/journal.pone.0261113",
"article-title": "Eculizumab in patients with severe coronavirus disease 2019 (COVID-19) requiring continuous positive airway pressure ventilator support: Retrospective cohort study",
"author": "Ruggenenti",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B41",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00297-1",
"article-title": "Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial",
"author": "Vlaar",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B42",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(23)00082-6",
"article-title": "Intravenous ravulizumab in mechanically ventilated patients hospitalised with severe COVID-19: a phase 3, multicentre, open-label, randomised controlled trial",
"author": "Annane",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "B43",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac984",
"article-title": "Three-day icatibant on top of standard care in patients with COVID-19 pneumonia (ICAT.COVID): a randomized, open-label, phase 2, proof-of-concept trial",
"author": "Malchair",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "B44",
"year": "2023"
},
{
"DOI": "10.3390/v13020309",
"article-title": "Safety and outcomes associated with the pharmacological inhibition of the kinin-kallikrein system in severe COVID-19",
"author": "Mansour",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B45",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1186/s13045-021-01177-0",
"article-title": "COVID-19 infection in adult patients with hematological Malignancies: a European Hematology Association Survey (EPICOVIDEHA)",
"author": "Pagano",
"doi-asserted-by": "publisher",
"first-page": "168",
"journal-title": "J Hematol Oncol",
"key": "B46",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.2500/aap.2013.34.3677",
"article-title": "Treatment with C1-esterase inhibitor concentrate in type I or II hereditary angioedema: a systematic literature review",
"author": "Bork",
"doi-asserted-by": "publisher",
"journal-title": "Allergy Asthma Proc",
"key": "B47",
"volume": "34",
"year": "2013"
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1255292/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Recombinant C1 inhibitor in the prevention of severe COVID-19: a randomized, open-label, multi-center phase IIa trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "14"
}