Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(22)00297-1, PANAMO, NCT04333420, Dec 2022
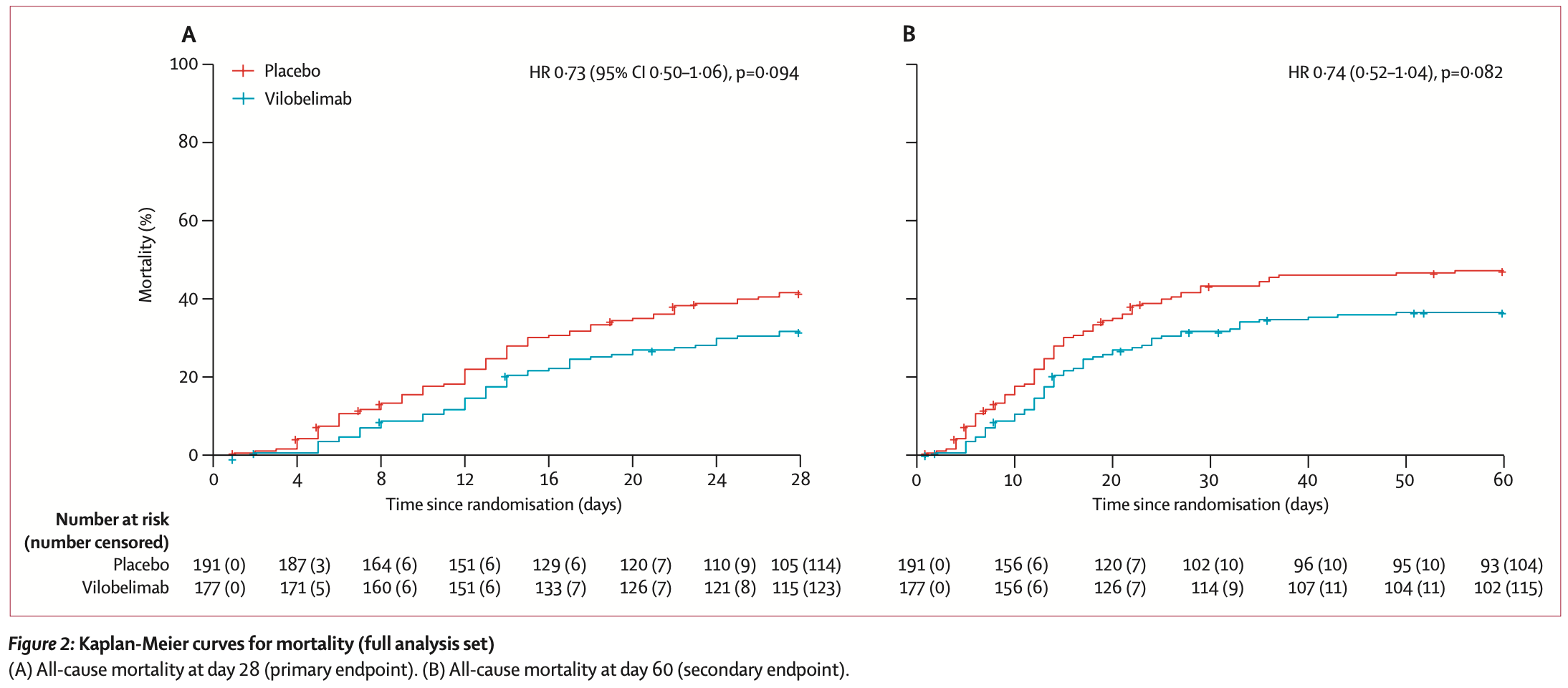
RCT 368 mechanically ventilated COVID-19 patients showing lower mortality with vilobelimab.
|
risk of death, 26.0% lower, HR 0.74, p = 0.08, treatment 177, control 191, day 60.
|
|
risk of death, 27.0% lower, HR 0.73, p = 0.09, treatment 177, control 191, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Vlaar et al., 31 Dec 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, median age 58.0, 109 authors, study period 1 October, 2020 - 4 October, 2021, trial NCT04333420 (history) (PANAMO).
Contact: a.p.vlaar@amsterdamumc.nl.
Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(22)00297-1
Background Vilobelimab, an anti-C5a monoclonal antibody, was shown to be safe in a phase 2 trial of invasively mechanically ventilated patients with COVID-19. Here, we aimed to determine whether vilobelimab in addition to standard of care improves survival outcomes in this patient population. Methods This randomised, double-blind, placebo-controlled, multicentre phase 3 trial was performed at 46 hospitals in the Netherlands, Germany, France, Belgium, Russia, Brazil, Peru, Mexico, and South Africa. Participants aged 18 years or older who were receiving invasive mechanical ventilation, but not more than 48 h after intubation at time of first infusion, had a PaO 2 /FiO 2 ratio of 60-200 mm Hg, and a confirmed SARS-CoV-2 infection with any variant in the past 14 days were eligible for this study. Eligible patients were randomly assigned (1:1) to receive standard of care and vilobelimab at a dose of 800 mg intravenously for a maximum of six doses (days 1, 2, 4, 8, 15, and 22) or standard of care and a matching placebo using permuted block randomisation. Treatment was not continued after hospital discharge. Participants, caregivers, and assessors were masked to group assignment. The primary outcome was defined as all-cause mortality at 28 days in the full analysis set (defined as all randomly assigned participants regardless of whether a patient started treatment, excluding patients randomly assigned in error) and measured using Kaplan-Meier analysis. Safety analyses included all patients who had received at least one infusion of either vilobelimab or placebo. This study is registered with ClinicalTrials.gov, NCT04333420.
Findings From Oct 1, 2020, to Oct 4, 2021, we included 368 patients in the ITT analysis (full analysis set; 177 in the vilobelimab group and 191 in the placebo group). One patient in the vilobelimab group was excluded from the primary analysis due to random assignment in error without treatment. At least one dose of study treatment was given to 364 (99%) patients (safety analysis set). 54 patients (31%) of 177 in the vilobelimab group and 77 patients (40%) of 191 in the placebo group died in the first 28 days. The all-cause mortality rate at 28 days was 32% (95% CI 25-39) in the vilobelimab group and 42% (35-49) in the placebo group (hazard ratio 0•73, 95% CI 0•50-1•06; p=0•094). In the predefined analysis without site-stratification, vilobelimab significantly reduced all-cause mortality at 28 days (HR 0•67, 95% CI 0•48-0•96; p=0•027). The most common TEAEs were acute kidney injury (35 [20%] of 175 in the vilobelimab group vs 40 [21%] of 189 in the placebo), pneumonia (38 [22%] vs 26 [14%]), and septic shock (24 [14%] vs 31 [16%]). Serious treatment-emergent adverse events were reported in 103 (59%) of 175 patients in the vilobelimab group versus 120 (63%) of 189 in the placebo group. Interpretation In addition to standard of care, vilobelimab improves survival of invasive mechanically ventilated patients with COVID-19 and leads to..
References
Afzali, Noris, Lambrecht, Kemper, The state of complement in COVID-19, Nat Rev Immunol
Aiello, Gastoldi, Galbusera, C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19, Blood Adv
Annane, Heming, Grimaldi-Bensouda, Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study, eClinicalMedicine
Carvelli, Demaria, Vély, Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis, Nature
Cyprian, Suleman, Abdelhafez, Complement C5a and clinical markers as predictors of COVID-19 disease severity and mortality in a multi-ethnic population, Front Immunol
De Bruin, Bos, Van Roon, Clinical features and prognostic factors in Covid-19: a prospective cohort study, eBioMedicine
Docherty, Harrison, Green, Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study, BMJ
Gordon, Mouncey, Al-Beidh, Interleukin-6 receptor antagonists in critically ill patients with COVID-19, N Engl J Med
Hilty, Keiser, Garcia, mRNA-based SARS-CoV-2 vaccination is associated with reduced ICU admission rate and disease severity in critically ill COVID-19 patients treated in Switzerland, Intensive Care Med
Jansen, Kompanje, Druml, Menon, Wiedermann et al., Deferred consent in emergency intensive care research: what if the patient dies early? Use the data or not?, Intensive Care Med
Levey, Stevens, Schmid, A new equation to estimate glomerular filtration rate, Ann Intern Med
Lim, Vlaar, De-Bruin, Brouwer, De Beek, Complement inhibition in severe COVID-19-blocking C5a seems to be key, eClinicalMedicine
Pocock, Stone, The nature of the p value, N Engl J Med
Ranieri, Rubenfeld, Thompson, Acute respiratory distress syndrome: the Berlin definition, JAMA
Riedemann, Habel, Ziereisen, Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition, Clin Immunol
Rosas, Bräu, Waters, Tocilizumab in hospitalized patients with severe COVID-19 pneumonia, N Engl J Med
Shah, Horo, In patients with COVID-19 receiving IMV or ECMO, adding baricitinib to usual care reduced all-cause mortality, Ann Intern Med
Shankar-Hari, Vale, Godolphin, Association between administration of il-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis, JAMA
Sun, Zhao, Liu, Treatment with anti-c5a antibody improves the outcome of H7N9 virus infection in African green monkeys, Clin Infect Dis
Thomas, Moreira, Jr, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months, N Engl J Med
Vlaar, De Bruin, Busch, Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial, Lancet Rheumatol
Vlaar, Lim, De Bruin, The anti-C5a antibody vilobelimab efficiently inhibits C5a in patients with severe COVID-19, Clin Transl Sci
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA
DOI record:
{
"DOI": "10.1016/s2213-2600(22)00297-1",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(22)00297-1",
"alternative-id": [
"S2213260022002971"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(22)00297-1"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(22)00365-4"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Vlaar",
"given": "Alexander P J",
"sequence": "first"
},
{
"affiliation": [],
"family": "Witzenrath",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Paassen",
"given": "Pieter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heunks",
"given": "Leo M A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourvillier",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Bruin",
"given": "Sanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Endry H T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brouwer",
"given": "Matthijs C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tuinman",
"given": "Pieter R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saraiva",
"given": "José F K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marx",
"given": "Gernot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lobo",
"given": "Suzana M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boldo",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simon-Campos",
"given": "Jesus A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cornet",
"given": "Alexander D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grebenyuk",
"given": "Anastasia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engelbrecht",
"given": "Johannes M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mukansi",
"given": "Murimisi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jorens",
"given": "Philippe G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zerbib",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rückinger",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pilz",
"given": "Korinna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Renfeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van de Beek",
"given": "Diederik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riedemann",
"given": "Niels C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vlaar",
"given": "Alexander P.J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Witzenrath",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Paassen",
"given": "Pieter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heunks",
"given": "Leo M.A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mourvillier",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Bruin",
"given": "Sanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Endry H.T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brouwer",
"given": "Matthijs C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tuinman",
"given": "Pieter R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saraiva",
"given": "José Francisco K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marx",
"given": "Gernot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lobo",
"given": "Suzana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boldo",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simon-Campos",
"given": "Jesus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cornet",
"given": "Alexander D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grebenyuk",
"given": "Anastasia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engelbrecht",
"given": "Johannes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mukansi",
"given": "Murimisi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jorens",
"given": "Philippe G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zerbib",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rückinger",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pilz",
"given": "Korinna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Renfeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van de Beek",
"given": "Diederik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riedemann",
"given": "Niels C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bulpa",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taccone",
"given": "Fabio S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hermans",
"given": "Greet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diltoer",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piagnerelli",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Neve",
"given": "Nikolaas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freire",
"given": "Antonio T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pizzol",
"given": "Felipe D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marinho",
"given": "Anna Karolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Victor H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arns da Cunha",
"given": "Clovis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neuville",
"given": "Mathilde",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dellamonica",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Annane",
"given": "Djillali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roquilly",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diehl",
"given": "Jean Luc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schneider",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mira",
"given": "Jean Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lascarrou",
"given": "Jean Baptiste",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desmedt",
"given": "Luc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupuis",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schwebel",
"given": "Carole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thiéry",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gründling",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berger",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Welte",
"given": "Tobias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bauer",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaschinski",
"given": "Ulrich",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matschke",
"given": "Klaus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercado-Longoria",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomez Quintana",
"given": "Belinda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamudio-Lerma",
"given": "Jorge Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno Hoyos Abril",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aleman Marquez",
"given": "Angel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pickkers",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Otterspoor",
"given": "Luuk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hercilla Vásquez",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seas Ramos",
"given": "Carlos Rafael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peña Villalobos",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gianella Malca",
"given": "Gonzalo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chávez",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Filimonov",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kulabukhov",
"given": "Vladimir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Acharya",
"given": "Pinak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Timmermans",
"given": "Sjoerd A.M.E.G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Busch",
"given": "Matthias H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Baarle",
"given": "Floor L.F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koning",
"given": "Rutger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ter Horst",
"given": "Liora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chekrouni",
"given": "Nora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Soest",
"given": "Thijs M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slim",
"given": "Marleen A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Vught",
"given": "Lonneke A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Amstel",
"given": "Rombout B.E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olie",
"given": "Sabine E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Zeggeren",
"given": "Ingeborg E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van de Poll",
"given": "Marcel C.G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thielert",
"given": "Claus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neukirchen",
"given": "Dorothee",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
7
]
],
"date-time": "2022-09-07T22:54:39Z",
"timestamp": 1662591279000
},
"deposited": {
"date-parts": [
[
2023,
5,
2
]
],
"date-time": "2023-05-02T06:48:19Z",
"timestamp": 1683010099000
},
"indexed": {
"date-parts": [
[
2024,
8,
22
]
],
"date-time": "2024-08-22T18:46:55Z",
"timestamp": 1724352415382
},
"is-referenced-by-count": 69,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002971?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002971?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1137-1146",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.ebiom.2021.103378",
"article-title": "Clinical features and prognostic factors in Covid-19: a prospective cohort study",
"author": "de Bruin",
"doi-asserted-by": "crossref",
"journal-title": "eBioMedicine",
"key": "10.1016/S2213-2600(22)00297-1_bib1",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00297-1_bib2",
"volume": "324",
"year": "2020"
},
{
"article-title": "Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study",
"author": "Docherty",
"journal-title": "BMJ",
"key": "10.1016/S2213-2600(22)00297-1_bib3",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/s41577-021-00665-1",
"article-title": "The state of complement in COVID-19",
"author": "Afzali",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Nat Rev Immunol",
"key": "10.1016/S2213-2600(22)00297-1_bib4",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2600-6",
"article-title": "Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis",
"author": "Carvelli",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(22)00297-1_bib5",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1182/bloodadvances.2021005246",
"article-title": "C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19",
"author": "Aiello",
"doi-asserted-by": "crossref",
"first-page": "866",
"journal-title": "Blood Adv",
"key": "10.1016/S2213-2600(22)00297-1_bib6",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1016/S2665-9913(20)30341-6",
"article-title": "Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial",
"author": "Vlaar",
"doi-asserted-by": "crossref",
"first-page": "e764",
"journal-title": "Lancet Rheumatol",
"key": "10.1016/S2213-2600(22)00297-1_bib7",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1111/cts.13213",
"article-title": "The anti-C5a antibody vilobelimab efficiently inhibits C5a in patients with severe COVID-19",
"author": "Vlaar",
"doi-asserted-by": "crossref",
"first-page": "854",
"journal-title": "Clin Transl Sci",
"key": "10.1016/S2213-2600(22)00297-1_bib8",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1007/s00134-007-0580-8",
"article-title": "Deferred consent in emergency intensive care research: what if the patient dies early? Use the data or not?",
"author": "Jansen",
"doi-asserted-by": "crossref",
"first-page": "894",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2213-2600(22)00297-1_bib9",
"volume": "33",
"year": "2007"
},
{
"DOI": "10.7326/0003-4819-150-9-200905050-00006",
"article-title": "A new equation to estimate glomerular filtration rate",
"author": "Levey",
"doi-asserted-by": "crossref",
"first-page": "604",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2213-2600(22)00297-1_bib10",
"volume": "150",
"year": "2009"
},
{
"article-title": "Acute respiratory distress syndrome: the Berlin definition",
"author": "Ranieri",
"first-page": "2526",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00297-1_bib11",
"volume": "307",
"year": "2012"
},
{
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with COVID-19",
"author": "Gordon",
"first-page": "1247",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00297-1_bib12",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.11330",
"article-title": "Association between administration of il-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis",
"author": "Shankar-Hari",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00297-1_bib13",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028700",
"article-title": "Tocilizumab in hospitalized patients with severe COVID-19 pneumonia",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1503",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00297-1_bib15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00297-1_bib16",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.7326/J22-0033",
"article-title": "In patients with COVID-19 receiving IMV or ECMO, adding baricitinib to usual care reduced all-cause mortality",
"author": "Shah",
"doi-asserted-by": "crossref",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2213-2600(22)00297-1_bib17",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2020.100590",
"article-title": "Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study",
"author": "Annane",
"doi-asserted-by": "crossref",
"journal-title": "eClinicalMedicine",
"key": "10.1016/S2213-2600(22)00297-1_bib18",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciu887",
"article-title": "Treatment with anti-c5a antibody improves the outcome of H7N9 virus infection in African green monkeys",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "586",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2213-2600(22)00297-1_bib19",
"volume": "60",
"year": "2015"
},
{
"DOI": "10.1016/j.eclinm.2021.100722",
"article-title": "Complement inhibition in severe COVID-19—blocking C5a seems to be key",
"author": "Lim",
"doi-asserted-by": "crossref",
"journal-title": "eClinicalMedicine",
"key": "10.1016/S2213-2600(22)00297-1_bib20",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2017.03.012",
"article-title": "Controlling the anaphylatoxin C5a in diseases requires a specifically targeted inhibition",
"author": "Riedemann",
"doi-asserted-by": "crossref",
"first-page": "25",
"journal-title": "Clin Immunol",
"key": "10.1016/S2213-2600(22)00297-1_bib21",
"volume": "180",
"year": "2017"
},
{
"DOI": "10.3389/fimmu.2021.707159",
"article-title": "Complement C5a and clinical markers as predictors of COVID-19 disease severity and mortality in a multi-ethnic population",
"author": "Cyprian",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/S2213-2600(22)00297-1_bib22",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2110345",
"article-title": "Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months",
"author": "Thomas",
"doi-asserted-by": "crossref",
"first-page": "1761",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00297-1_bib24",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1007/s00134-021-06610-z",
"article-title": "mRNA-based SARS-CoV-2 vaccination is associated with reduced ICU admission rate and disease severity in critically ill COVID-19 patients treated in Switzerland",
"author": "Hilty",
"doi-asserted-by": "crossref",
"first-page": "362",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2213-2600(22)00297-1_bib25",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc1612970",
"article-title": "The nature of the p value",
"author": "Pocock",
"doi-asserted-by": "crossref",
"first-page": "2205",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00297-1_bib27",
"volume": "375",
"year": "2016"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260022002971"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "10"
}