Real-world clinical outcomes of treatment with molnupiravir for patients with mild- to-moderate coronavirus disease 2019 during the Omicron variant pandemic
et al., Research Square, doi:10.21203/rs.3.rs-2118653/v1, Oct 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 1,921 patients in Japan, showing no significant difference in progression with sotrovimab use.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of progression, 8.3% higher, OR 1.08, p = 0.73, treatment 672, control 1,257, adjusted per study, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Suzuki et al., 5 Oct 2022, retrospective, Japan, preprint, 53 authors.
Contact: shibatay@fmu.ac.jp.
Real-world clinical outcomes of treatment with molnupiravir for patients with mild- to-moderate coronavirus disease 2019 during the Omicron variant pandemic
doi:10.21203/rs.3.rs-2118653/v1
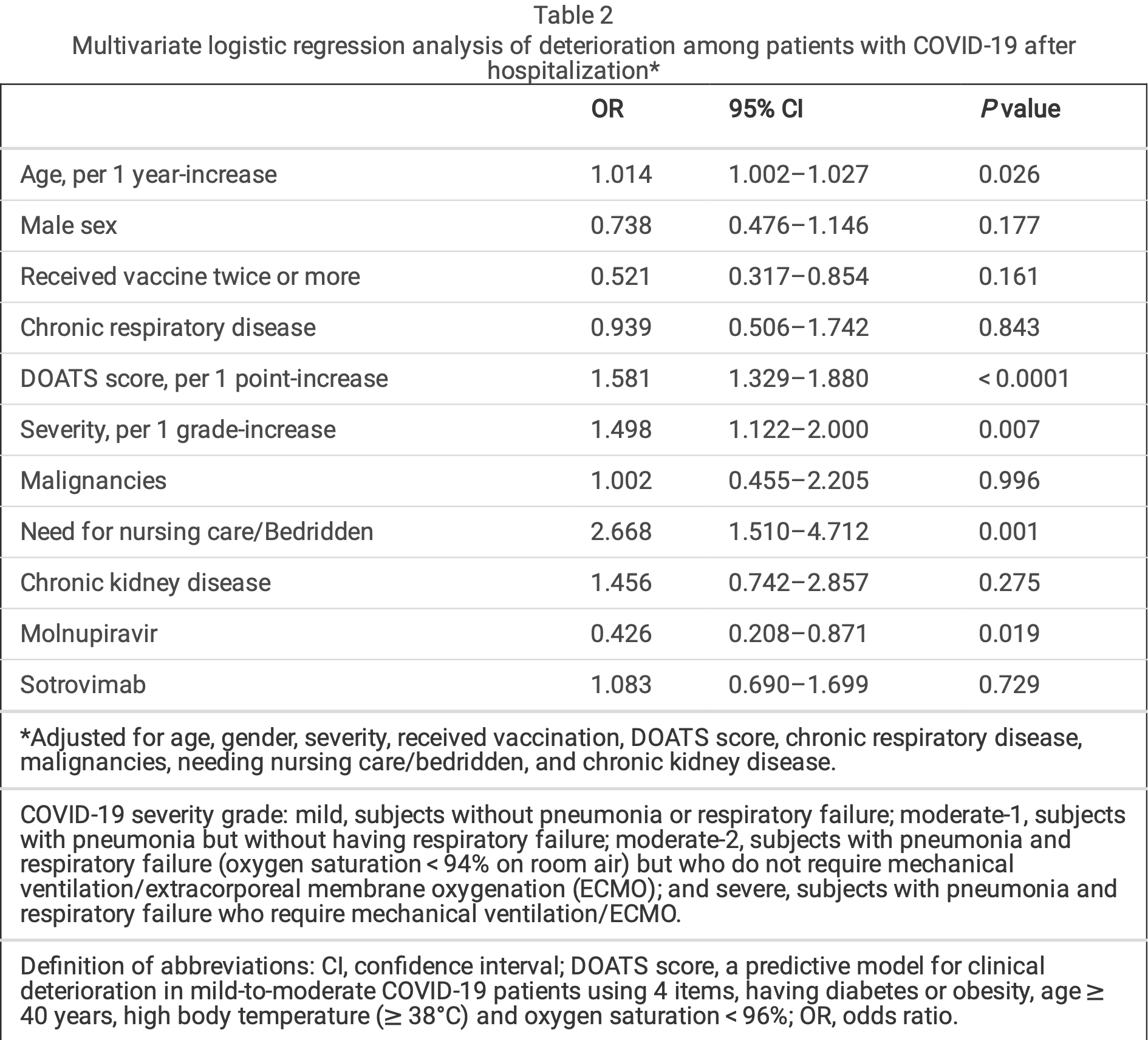
Background It is unclear whether molnupiravir has a bene cial effect on vaccinated patients infected with the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We here evaluated the e cacy of molnupiravir in patients with mild-to-moderate coronavirus disease 2019 (COVID-19) during the Omicron variant surge in Fukushima Prefecture, Japan.
Methods We enrolled patients with mild-to-moderate COVID-19 who were admitted to hospitals between January and April, 2022. Clinical deterioration after admission was compared between molnupiravir users (n = 281) and non-users (n = 1,636).
Results The molnupiravir users were older (P < 0.0001), and had greater rates of history of chronic respiratory disease (P = 0.039), hypertension (P < 0.0001), dyslipidemia (P < 0.0001), diabetes mellitus (P < 0.0001), and cardiac disease (P = 0.003) than the non-users. The clinical deterioration rate was signi cantly lower in the molnupiravir users compared to the non-users (3.92% vs 7.46%; P = 0.021). Multivariate logistic regression analysis demonstrated that receiving molnupiravir was a factor for preventing deterioration (odds ratio 0.426; 95% con dence interval 0.208-0.871; P = 0.019), independent of receiving the SARS-CoV-2 vaccine. Furthermore, in 259 patients who were selected from each group after matching on the propensity score, the rate of deterioration was signi cantly lower among those receiving molnupiravir compared to those not receiving molnupiravir (3.86% vs 9.65%; p = 0.008).
Conclusion This real-world study demonstrates that molnupiravir contributes to the prevention of deterioration in COVID-19 patients after hospitalization during the Omicron variant phase.
Author contribution Conception and design: Yasuhito Suzuki and Yoko Shibata. Analysis and drafting the manuscript: Yasuhito Suzuki and Yoko Shibata. Data curation: all authors. Final approval of the manuscript: all authors.
Ethic This study was performed in line with the principles of the Declaration of Helsinki. The protocol was approved by the local ethical committee.
Consent to publication The authors seen the nal version of the manuscript and approved submission for publication.
References
Aggarwal, Beaty, Bennett, Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients, J Infect Dis, doi:10.1093/infdis/jiac206
Austin, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharm Stat, doi:10.1002/pst.433
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bojkova, Widera, Ciesek, Wass, Michaelis et al., Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates, Cell Res, doi:10.1038/s41422-022-00619-9
Callaway, Heavily mutated Omicron variant puts scientists on alert, Nature, doi:10.1038/d41586-021-03552-w
Carlos, Wong, Lau, Lau, Cowling et al., Realworld effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00507-2
Gupta, Gonzalez-Rojas, Juarez, Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, Jama, doi:10.1001/jama.2022.2832
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hirotsu, Maejima, Shibusawa, SARS-CoV-2 Omicron sublineage BA.2 replaces BA.1.1: Genomic surveillance in Japan from September 2021 to March 2022, J Infect, doi:10.1016/j.jinf.2022.04.040
Hoffmann, Krüger, Schulz, The Omicron variant is highly resistant against antibodymediated neutralization: Implications for control of the COVID-19 pandemic, Cell, doi:10.1016/j.cell.2021.12.032
Korber, Fischer, Gnanakaran, Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus, Cell, doi:10.1016/j.cell.2020.06.043
Liu, Rocklöv, The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta, J Travel Med, doi:10.1093/jtm/taac037
Ohashi, Hishiki, Akazawa, Different e cacies of neutralizing antibodies and antiviral drugs on SARS-CoV-2 Omicron subvariants, BA.1 and BA.2 Antiviral Res, doi:10.1016/j.antiviral.2022.105372
Ong, Ren, Lee, Real-World Use of Sotrovimab for Pre-Emptive Treatment in High-Risk Hospitalized COVID-19 Patients: An Observational Cross-Sectional Study, Antibiotics, doi:10.3390/antibiotics11030345
Polack, Thomas, Kitchin, Safety and E cacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Ren, Nishimura, Tjan, Large-scale serosurveillance of COVID-19 in Japan: Acquisition of neutralizing antibodies for Delta but not for Omicron and requirement of booster vaccination to overcome the Omicron's outbreak, PLoS One, doi:10.1371/journal.pone.0266270
Rosenke, Okumura, Lewis, MK-4482) is e cacious against Omicron and other SARS-CoV-2 variants in the Syrian hamster COVID-19 model, doi:10.1101/2022.02.22.481491v1
Shiba, Kawahara, Using Propensity Scores for Causal Inference: Pitfalls and Tips, J Epidemiol, doi:10.2188/jea.JE20210145
Shibata, Minemura, Suzuki, Development and external validation of the DOAT and DOATS scores: simple decision support tools to identify disease progression among nonelderly patients with mild/moderate COVID-19, doi:10.1101/2021.12.13.21267698v1
Takashita, Kinoshita, Yamayoshi, E cacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Vanblargan, Errico, Halfmann, An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med, doi:10.1038/s41591-021-01678-y
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Yamamoto, Wada, Ichikawa, Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection, J Clin Med, doi:10.3390/jcm10173775
DOI record:
{
"DOI": "10.21203/rs.3.rs-2118653/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-2118653/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Background\n It is unclear whether molnupiravir has a beneficial effect on vaccinated patients infected with the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We here evaluated the efficacy of molnupiravir in patients with mild-to-moderate coronavirus disease 2019 (COVID-19) during the Omicron variant surge in Fukushima Prefecture, Japan.\nMethods\n We enrolled patients with mild-to-moderate COVID-19 who were admitted to hospitals between January and April, 2022. Clinical deterioration after admission was compared between molnupiravir users (n = 281) and non-users (n = 1,636).\nResults\n The molnupiravir users were older (P < 0.0001), and had greater rates of history of chronic respiratory disease (P = 0.039), hypertension (P < 0.0001), dyslipidemia (P < 0.0001), diabetes mellitus (P < 0.0001), and cardiac disease (P = 0.003) than the non-users. The clinical deterioration rate was significantly lower in the molnupiravir users compared to the non-users (3.92% vs 7.46%; P = 0.021). Multivariate logistic regression analysis demonstrated that receiving molnupiravir was a factor for preventing deterioration (odds ratio 0.426; 95% confidence interval 0.208–0.871; P = 0.019), independent of receiving the SARS-CoV-2 vaccine. Furthermore, in 259 patients who were selected from each group after matching on the propensity score, the rate of deterioration was significantly lower among those receiving molnupiravir compared to those not receiving molnupiravir (3.86% vs 9.65%; p = 0.008).\nConclusion\n This real-world study demonstrates that molnupiravir contributes to the prevention of deterioration in COVID-19 patients after hospitalization during the Omicron variant phase.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
9,
30
]
]
},
"author": [
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Suzuki",
"given": "Yasuhito",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Shibata",
"given": "Yoko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Minemura",
"given": "Hiroyuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Nikaido",
"given": "Takefumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Tanino",
"given": "Yoshinori",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ohara General Hospital"
}
],
"family": "Fukuhara",
"given": "Atsuro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Red Cross Hospital"
}
],
"family": "Kanno",
"given": "Ryuzo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fujita General Hospital"
}
],
"family": "Saito",
"given": "Hiroyuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fujita General Hospital"
}
],
"family": "Suzuki",
"given": "Shuzo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Saiseikai Fukushima General Hospital"
}
],
"family": "Inokoshi",
"given": "Yayoi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Sando",
"given": "Eiichiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Saiseikai Kawamata Hospital"
}
],
"family": "Sakuma",
"given": "Hirofumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aizu Chuo Hospital"
}
],
"family": "Kobayashi",
"given": "Tatsuho",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Kume",
"given": "Hiroaki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Takeda General Hospital"
}
],
"family": "Kamimoto",
"given": "Masahiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bange Kousei General Hospital"
}
],
"family": "Aoki",
"given": "Hideko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Yurin Hospital"
}
],
"family": "Takama",
"given": "Akira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Yurin Hospital"
}
],
"family": "Iizuka",
"given": "Taku",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Iwaki City Medical Center"
}
],
"family": "Kamiyama",
"given": "Takamichi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kashima Hospital"
}
],
"family": "Nakayama",
"given": "Masaru",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Rosai Hospital"
}
],
"family": "Saito",
"given": "Kiyoshi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Futaba Medical Center"
}
],
"family": "Tanigawa",
"given": "Koichi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Soma General Hospital"
}
],
"family": "Sato",
"given": "Masahiko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Soma General Hospital"
}
],
"family": "Waragai",
"given": "Yuichi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Minamisoma Municipal General Hospital"
}
],
"family": "Kambe",
"given": "Toshiyuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Onahama Chuo Clinic"
}
],
"family": "Kanzaki",
"given": "Norio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shirakawa Satellite for Teaching and Research, Fukushima Medical University"
}
],
"family": "Azuma",
"given": "Teruhisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shirakawa Kosei Hospital"
}
],
"family": "Okamoto",
"given": "Hiromasa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hoshi General Hospital"
}
],
"family": "Sakamoto",
"given": "Keiji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hoshi General Hospital"
}
],
"family": "Nakamura",
"given": "Yuichi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Iwase General Hospital"
}
],
"family": "Ohtani",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Southern TOHOKU General Hospital"
}
],
"family": "Waragai",
"given": "Mitsuru",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jusendo General Hospital"
}
],
"family": "Maeda",
"given": "Shinsaku",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ohta Nishinouchi Hospital"
}
],
"family": "Ishida",
"given": "Tokiya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tsuboi Hospital"
}
],
"family": "Sugino",
"given": "Keishi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Wakamatsu Infection Leading Clinic"
}
],
"family": "Abe",
"given": "Wataru",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Tsukada",
"given": "Yasuhiko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Lee",
"given": "Tomoyoshi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Yamada",
"given": "Ryuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Sato",
"given": "Riko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Onuma",
"given": "Takumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Tomita",
"given": "Hikaru",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Saito",
"given": "Mikako",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Watanabe",
"given": "Natsumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Rikimaru",
"given": "Mami",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Kawamata",
"given": "Takaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Morimoto",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Togawa",
"given": "Ryuichi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Sato",
"given": "Yuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Saito",
"given": "Junpei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Kanazawa",
"given": "Kenya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Hamaguchi",
"given": "Sugihiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fukushima Medical University"
}
],
"family": "Iseki",
"given": "Ken",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
5
]
],
"date-time": "2022-10-05T18:37:20Z",
"timestamp": 1664995040000
},
"deposited": {
"date-parts": [
[
2022,
10,
5
]
],
"date-time": "2022-10-05T18:37:26Z",
"timestamp": 1664995046000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2022,
10,
5
]
],
"date-time": "2022-10-05T19:12:35Z",
"timestamp": 1664997155368
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
5
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
5
]
],
"date-time": "2022-10-05T00:00:00Z",
"timestamp": 1664928000000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-2118653/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-2118653/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
10,
5
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2022,
10,
5
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19",
"author": "Weinreich DM",
"doi-asserted-by": "publisher",
"first-page": "238",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "ref1",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med 2021; 384(3):238–51. https://doi.org/10.1056/NEJMoa2035002.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial",
"author": "Gupta A",
"doi-asserted-by": "publisher",
"first-page": "1236",
"journal-title": "Jama",
"key": "ref2",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. Jama 2022; 327:1236–46. https://doi.org/10.1001/jama.2022.2832.",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal A",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "ref3",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022; 386:509–20. https://doi.org/10.1056/NEJMoa2116044.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond J",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "ref4",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022; 386:1397–408. https://doi.org/10.1056/NEJMoa2118542.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"article-title": "Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus",
"author": "Korber B",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "ref5",
"unstructured": "Korber B, Fischer WM, Gnanakaran S, et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020; 182:812 – 27.e19. https://doi.org/10.1016/j.cell.2020.06.043.",
"year": "2020"
},
{
"DOI": "10.1038/d41586-021-03552-w",
"article-title": "Heavily mutated Omicron variant puts scientists on alert",
"author": "Callaway E",
"doi-asserted-by": "publisher",
"first-page": "21",
"journal-title": "Nature",
"key": "ref6",
"unstructured": "Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021; 600:21. https://doi.org/10.1038/d41586-021-03552-w.",
"volume": "600",
"year": "2021"
},
{
"DOI": "10.1093/jtm/taac037",
"article-title": "The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta",
"author": "Liu Y",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "J Travel Med",
"key": "ref7",
"unstructured": "Liu Y, Rocklöv J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J Travel Med 2022; 29. https://doi.org/10.1093/jtm/taac037.",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0266270",
"article-title": "Large-scale serosurveillance of COVID-19 in Japan: Acquisition of neutralizing antibodies for Delta but not for Omicron and requirement of booster vaccination to overcome the Omicron's outbreak",
"author": "Ren Z",
"doi-asserted-by": "publisher",
"first-page": "e0266270",
"journal-title": "PLoS One",
"key": "ref8",
"unstructured": "Ren Z, Nishimura M, Tjan LH, et al. Large-scale serosurveillance of COVID-19 in Japan: Acquisition of neutralizing antibodies for Delta but not for Omicron and requirement of booster vaccination to overcome the Omicron's outbreak. PLoS One 2022; 17:e0266270. https://doi.org/10.1371/journal.pone.0266270.",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2021.12.032",
"article-title": "The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic",
"author": "Hoffmann M",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "ref9",
"unstructured": "Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022; 185:447 – 56.e11. https://doi.org/10.1016/j.cell.2021.12.032.",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies",
"author": "VanBlargan LA",
"doi-asserted-by": "publisher",
"first-page": "490",
"journal-title": "Nat Med",
"key": "ref10",
"unstructured": "VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med 2022; 28:490–5. https://doi.org/10.1038/s41591-021-01678-y.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study",
"author": "Carlos KH",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "ref11",
"unstructured": "Carlos K.H. Wong ICHA, Kristy T.K. Lau, Eric H. Y. Lau, Benjamin J. Cowling, Gabriel M. Leung. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis 2022. https://doi.org/10.1016/S1473-3099(22)00507-2.",
"year": "2022"
},
{
"key": "ref12",
"unstructured": "The official website of Fukushima Prefecture. The variant of COVID-19 in Fukushima from January 2022 (in Japanese). https://www.pref.fukushima.lg.jp/site/covid19-portal/variant.html. Accessed 1 June 2022."
},
{
"key": "ref13",
"unstructured": "Japan Ministry of Health Labor and Welfare. Clinical Management of patients with COVID-19: A guide for front-line healthcare workers (in Japanese). Available at: https://www.niph.go.jp/h-crisis/wp-content/uploads/2020/07/20200706103735_content_000646531.pdf. Accessed 1 June 2022."
},
{
"DOI": "10.3390/jcm10173775",
"article-title": "Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection",
"author": "Yamamoto A",
"doi-asserted-by": "publisher",
"first-page": "3775",
"journal-title": "J Clin Med",
"key": "ref14",
"unstructured": "Yamamoto A, Wada H, Ichikawa Y, et al. Evaluation of Biomarkers of Severity in Patients with COVID-19 Infection. J Clin Med 2021; 10:3775. https://doi.org/10.3390/jcm10173775.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1101/2021.12.13.21267698v1",
"author": "Shibata Y",
"doi-asserted-by": "publisher",
"key": "ref15",
"unstructured": "Shibata Y, Minemura H, Suzuki Y, et al. Development and external validation of the DOAT and DOATS scores: simple decision support tools to identify disease progression among nonelderly patients with mild/moderate COVID-19. medRxiv [Preprint] December 14 2021. Available from:ãhttps://www.medrxiv.org/content/10.1101/2021.12.13.21267698v1."
},
{
"DOI": "10.2188/jea.JE20210145",
"article-title": "Using Propensity Scores for Causal Inference: Pitfalls and Tips",
"author": "Shiba K",
"doi-asserted-by": "publisher",
"journal-title": "J Epidemiol",
"key": "ref16",
"unstructured": "Shiba K, Kawahara T. Using Propensity Scores for Causal Inference: Pitfalls and Tips. J Epidemiol 2021; 31:457 – 63. https://doi.org/10.2188/jea.JE20210145.",
"year": "2021"
},
{
"DOI": "10.1002/pst.433",
"article-title": "Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies",
"author": "Austin PC",
"doi-asserted-by": "publisher",
"first-page": "150",
"journal-title": "Pharm Stat",
"key": "ref17",
"unstructured": "Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–61. https://doi.org/10.1002/pst.433.",
"volume": "10",
"year": "2011"
},
{
"DOI": "10.1038/s41422-022-00619-9",
"article-title": "Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates",
"author": "Bojkova D",
"doi-asserted-by": "publisher",
"first-page": "319",
"journal-title": "Cell Res",
"key": "ref18",
"unstructured": "Bojkova D, Widera M, Ciesek S, Wass MN, Michaelis M, Cinatl J Jr. Reduced interferon antagonism but similar drug sensitivity in Omicron variant compared to Delta variant of SARS-CoV-2 isolates. Cell Res 2022: 32:319–21. https://doi.org/10.1038/s41422-022-00619-9.",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2",
"author": "Takashita E",
"doi-asserted-by": "publisher",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "ref19",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. N Engl J Med 2022; 386:1475–7. https://doi.org/10.1056/NEJMc2201933.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2022.105372",
"article-title": "Different efficacies of neutralizing antibodies and antiviral drugs on SARS-CoV-2 Omicron subvariants, BA.1 and BA.2 Antiviral Res",
"author": "Ohashi H",
"doi-asserted-by": "publisher",
"key": "ref20",
"unstructured": "Ohashi H, Hishiki T, Akazawa D, et al. Different efficacies of neutralizing antibodies and antiviral drugs on SARS-CoV-2 Omicron subvariants, BA.1 and BA.2 Antiviral Res. 2022. https://doi.org/10.1016/j.antiviral.2022.105372.",
"year": "2022"
},
{
"DOI": "10.1101/2022.02.22.481491v1",
"author": "Rosenke K",
"doi-asserted-by": "publisher",
"key": "ref21",
"unstructured": "Rosenke K, Okumura A, Lewis MC, et al. Molnupiravir (MK-4482) is efficacious against Omicron and other SARS-CoV-2 variants in the Syrian hamster COVID-19 model. medRxiv [Preprint] February 24 2022 Available from: https://www.biorxiv.org/content/10.1101/2022.02.22.481491v1."
},
{
"DOI": "10.3390/antibiotics11030345",
"article-title": "Real-World Use of Sotrovimab for Pre-Emptive Treatment in High-Risk Hospitalized COVID-19 Patients: An Observational Cross-Sectional Study",
"author": "Ong SWX",
"doi-asserted-by": "publisher",
"first-page": "345",
"journal-title": "Antibiotics (Basel)",
"key": "ref22",
"unstructured": "Ong SWX, Ren D, Lee PH, et al. Real-World Use of Sotrovimab for Pre-Emptive Treatment in High-Risk Hospitalized COVID-19 Patients: An Observational Cross-Sectional Study. Antibiotics (Basel) 2022; 11:345. https://doi.org/10.3390/antibiotics11030345.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac206",
"article-title": "Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients",
"author": "Aggarwal NR",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Dis",
"key": "ref23",
"unstructured": "Aggarwal NR, Beaty LE, Bennett TD, et al. Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients. J Infect Dis 2022; 16;jiac206. https://doi.org/10.1093/infdis/jiac206.",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.04.040",
"article-title": "SARS-CoV-2 Omicron sublineage BA.2 replaces BA.1.1: Genomic surveillance in Japan from September 2021 to March 2022",
"author": "Hirotsu Y",
"doi-asserted-by": "publisher",
"first-page": "174",
"journal-title": "J Infect",
"key": "ref24",
"unstructured": "Hirotsu Y, Maejima M, Shibusawa M, et al. SARS-CoV-2 Omicron sublineage BA.2 replaces BA.1.1: Genomic surveillance in Japan from September 2021 to March 2022. J Infect 2022; 85:174–211. https://doi.org/10.1016/j.jinf.2022.04.040.",
"volume": "85",
"year": "2022"
},
{
"key": "ref25",
"unstructured": "The proportion of SARS-CoV-2 Omicron Subvariant BA.2 in Fukushima in April 2022 (in Japanese). https://www.minpo.jp/news/moredetail/2022041296103. Accessed 1 June 2022."
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine",
"author": "Polack FP",
"doi-asserted-by": "publisher",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "ref26",
"unstructured": "Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020; 383:2603–15. https://doi.org/10.1056/NEJMoa2034577.",
"volume": "383",
"year": "2020"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-2118653/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Real-world clinical outcomes of treatment with molnupiravir for patients with mild- to-moderate coronavirus disease 2019 during the Omicron variant pandemic",
"type": "posted-content"
}
suzuki2-pp
