Real-World Use of Sotrovimab for Pre-Emptive Treatment in High-Risk Hospitalized COVID-19 Patients: An Observational Cross-Sectional Study
et al., Antibiotics, doi:10.3390/antibiotics11030345, Mar 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 19 sotrovimab patients and 75 controls is Singapore, showing lower progression with treatment.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
|
risk of death, 60.5% lower, RR 0.39, p = 0.45, treatment 1 of 19 (5.3%), control 10 of 75 (13.3%), NNT 12.
|
|
risk of ICU admission, 56.1% lower, RR 0.44, p = 0.35, treatment 2 of 19 (10.5%), control 18 of 75 (24.0%), NNT 7.4.
|
|
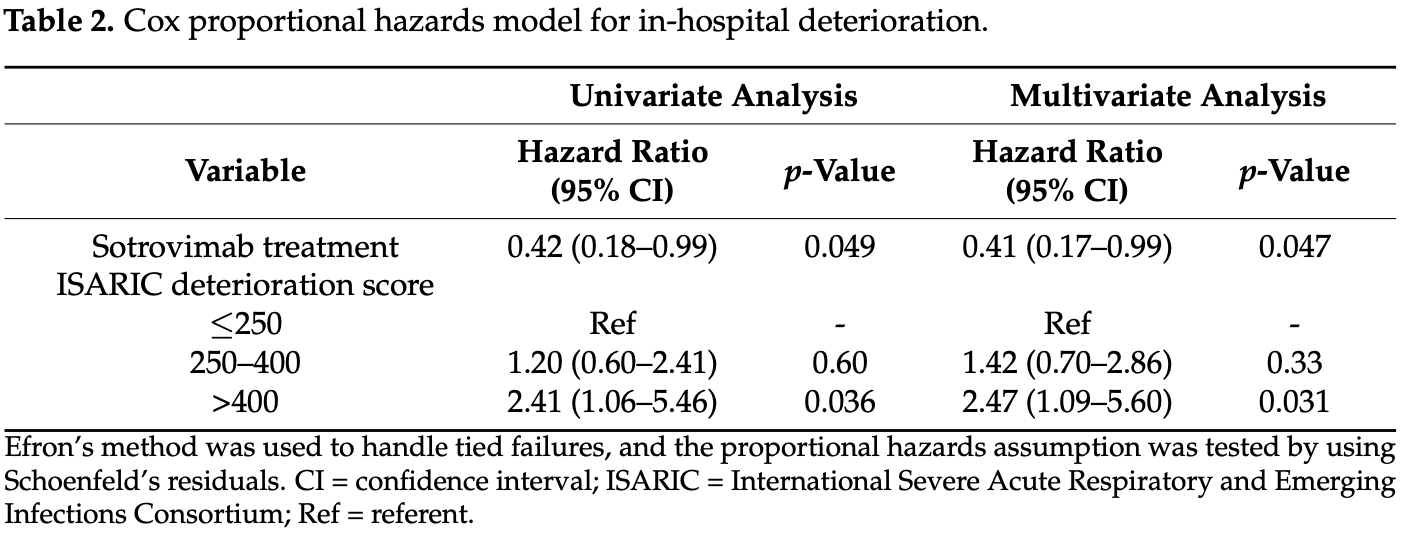
risk of progression, 59.0% lower, HR 0.41, p = 0.047, treatment 19, control 75, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Ong et al., 5 Mar 2022, retrospective, Singapore, peer-reviewed, 10 authors, average treatment delay 2.0 days.
Real-World Use of Sotrovimab for Pre-Emptive Treatment in High-Risk Hospitalized COVID-19 Patients: An Observational Cross-Sectional Study
Antibiotics, doi:10.3390/antibiotics11030345
Data on use of monoclonal antibodies (mAbs) in hospitalized patients are limited. In this cross-sectional study, we evaluated the use of mAbs for early treatment of unvaccinated hospitalized patients with mild-to-moderate COVID-19. All inpatients at our center were screened on 27 October 2021. Primary outcome was in-hospital deterioration as defined by a composite of oxygen requirement, intensive care unit (ICU) admission, or mortality within 28 days of admission. Ninety-four out of 410 COVID-19 inpatients were included in the final analysis, of whom 19 (20.2%) received early treatment with sotrovimab. The median age was 73 years (IQR 61-83), and 35 (37.2%) were female. Although the treatment group was significantly older and had more comorbidities, there was a lower proportion of progression to oxygen requirement (31.6% vs. 54.7%), ICU admission (10.5% vs. 24.0%), or mortality (5.3% vs. 13.3%). Kaplan-Meier curves showed a significant difference in time to in-hospital deterioration (log-rank test, p = 0.043). Cox proportional hazards model for in-hospital deterioration showed that sotrovimab treatment was protective (hazard ratio, 0.41; 95% CI, 0.17-0.99; p = 0.047) after adjustment for baseline ISARIC deterioration score. Our findings support the use of sotrovimab for early treatment in hospitalized patients with mild-to-moderate COVID-19 at a high risk of disease progression.
Informed Consent Statement: Patient consent was waived as approved by the institutional ethics committee for retrospective data collection without collection of individually identifiable patient identifiers.
Data Availability Statement: The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest: BEY reports personal fees from Roche and Sanofi outside the submitted work. All other authors declare no competing interest.
References
Bierle, Ganesh, Tulledge-Scheitel, Hanson, Arndt et al., Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities, J. Infect. Dis, doi:10.1093/infdis/jiab570
Chemaitelly, Tang, Hasan, Al Mukdad, Yassine et al., Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar, N. Engl. J. Med, doi:10.1056/NEJMoa2114114
Dagan, Barda, Kepten, Miron, Perchik et al., BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting, N. Engl. J. Med, doi:10.1056/NEJMoa2101765
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2102685
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N. Engl. J. Med, doi:10.1056/NEJMoa2107934
Gupta, Harrison, Ho, Docherty, Knight et al., Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: A prospective cohort study, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30559-2
Iketani, Liu, Guo, Liu, Huang et al., Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages, bioRxiv, doi:10.1038/s41586-022-04594-4
Kreuzberger, Hirsch, Chai, Tomlinson, Khosravi et al., SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst. Rev
Lundgren, Grund, A Neutralizing Monoclonal Antibody for Hospitalized Patients with COVID-19, N. Engl. J. Med
Ng, Koh, Chiew, Marimuthu, Thevasagayam et al., Impact of Delta Variant and Vaccination on SARS-CoV-2 Secondary Attack Rate Among Household Close Contacts, Lancet Reg. Health West. Pac, doi:10.1016/j.lanwpc.2021.100299
Ong, Sutjipto, Lee, Dugan, Khoo et al., Validation of ISARIC 4C mortality and deterioration scores in a mixed vaccination status cohort of hospitalized COVID-19 patients in Singapore, Clin. Infect. Dis, doi:10.1093/cid/ciac087
Tartof, Slezak, Fischer, Hong, Ackerson et al., Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study, Lancet, doi:10.1016/S0140-6736(21)02183-8
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV Antibody Combination and Outcomes in Outpatients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2108163
Wolter, Jassat, Walaza, Welch, Moultrie et al., Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study, Lancet, doi:10.1016/S0140-6736(22)00017-4
Young, Fong, Chang, Tan, Rouers et al., Comparison of the Clinical Features, viral Shedding and Immune Response in Vaccine Breakthrough Infection by the Omicron and Delta Variants
Zitek, Jodoin, Kheradia, Napolillo, Dalley et al., Vaccinated patients have reduced rates of hospitalization after receiving casirivimab and imdevimab for COVID-19, Am. J. Emerg. Med, doi:10.1016/j.ajem.2021.10.044
DOI record:
{
"DOI": "10.3390/antibiotics11030345",
"ISSN": [
"2079-6382"
],
"URL": "http://dx.doi.org/10.3390/antibiotics11030345",
"abstract": "<jats:p>Data on use of monoclonal antibodies (mAbs) in hospitalized patients are limited. In this cross-sectional study, we evaluated the use of mAbs for early treatment of unvaccinated hospitalized patients with mild-to-moderate COVID-19. All inpatients at our center were screened on 27 October 2021. Primary outcome was in-hospital deterioration as defined by a composite of oxygen requirement, intensive care unit (ICU) admission, or mortality within 28 days of admission. Ninety-four out of 410 COVID-19 inpatients were included in the final analysis, of whom 19 (20.2%) received early treatment with sotrovimab. The median age was 73 years (IQR 61–83), and 35 (37.2%) were female. Although the treatment group was significantly older and had more comorbidities, there was a lower proportion of progression to oxygen requirement (31.6% vs. 54.7%), ICU admission (10.5% vs. 24.0%), or mortality (5.3% vs. 13.3%). Kaplan–Meier curves showed a significant difference in time to in-hospital deterioration (log-rank test, p = 0.043). Cox proportional hazards model for in-hospital deterioration showed that sotrovimab treatment was protective (hazard ratio, 0.41; 95% CI, 0.17–0.99; p = 0.047) after adjustment for baseline ISARIC deterioration score. Our findings support the use of sotrovimab for early treatment in hospitalized patients with mild-to-moderate COVID-19 at a high risk of disease progression.</jats:p>",
"alternative-id": [
"antibiotics11030345"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8570-436X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ong",
"given": "Sean W. X.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ren",
"given": "Dongdong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Pei Hua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sutjipto",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dugan",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khoo",
"given": "Bo Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tay",
"given": "Jun Xin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vasoo",
"given": "Shawn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Young",
"given": "Barnaby E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lye",
"given": "David C.",
"sequence": "additional"
}
],
"container-title": [
"Antibiotics"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
7
]
],
"date-time": "2022-03-07T01:35:50Z",
"timestamp": 1646616950000
},
"deposited": {
"date-parts": [
[
2022,
3,
7
]
],
"date-time": "2022-03-07T15:28:51Z",
"timestamp": 1646666931000
},
"indexed": {
"date-parts": [
[
2022,
3,
7
]
],
"date-time": "2022-03-07T16:14:41Z",
"timestamp": 1646669681621
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2079-6382"
}
],
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3,
5
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
5
]
],
"date-time": "2022-03-05T00:00:00Z",
"timestamp": 1646438400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2079-6382/11/3/345/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "345",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
5
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMoa2101765",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1056/NEJMoa2102685",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A Neutralizing Monoclonal Antibody for Hospitalized Patients with COVID-19",
"author": "Lundgren",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "N. Engl. J. Med.",
"key": "ref5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)00163-5",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1016/S2213-2600(20)30559-2",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1093/cid/ciac087",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1016/j.lanwpc.2021.100299",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"author": "Young",
"key": "ref11",
"series-title": "Comparison of the Clinical Features, viral Shedding and Immune Response in Vaccine Breakthrough Infection by the Omicron and Delta Variants",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00017-4",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/S0140-6736(21)02183-8",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1056/NEJMoa2114114",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.ajem.2021.10.044",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1093/infdis/jiab570",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"article-title": "SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19",
"author": "Kreuzberger",
"first-page": "CD013825",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref18",
"volume": "9",
"year": "2021"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2079-6382/11/3/345"
}
},
"score": 1,
"short-container-title": [
"Antibiotics"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Pharmacology, Toxicology and Pharmaceutics",
"Biochemistry",
"Microbiology"
],
"subtitle": [],
"title": [
"Real-World Use of Sotrovimab for Pre-Emptive Treatment in High-Risk Hospitalized COVID-19 Patients: An Observational Cross-Sectional Study"
],
"type": "journal-article",
"volume": "11"
}
