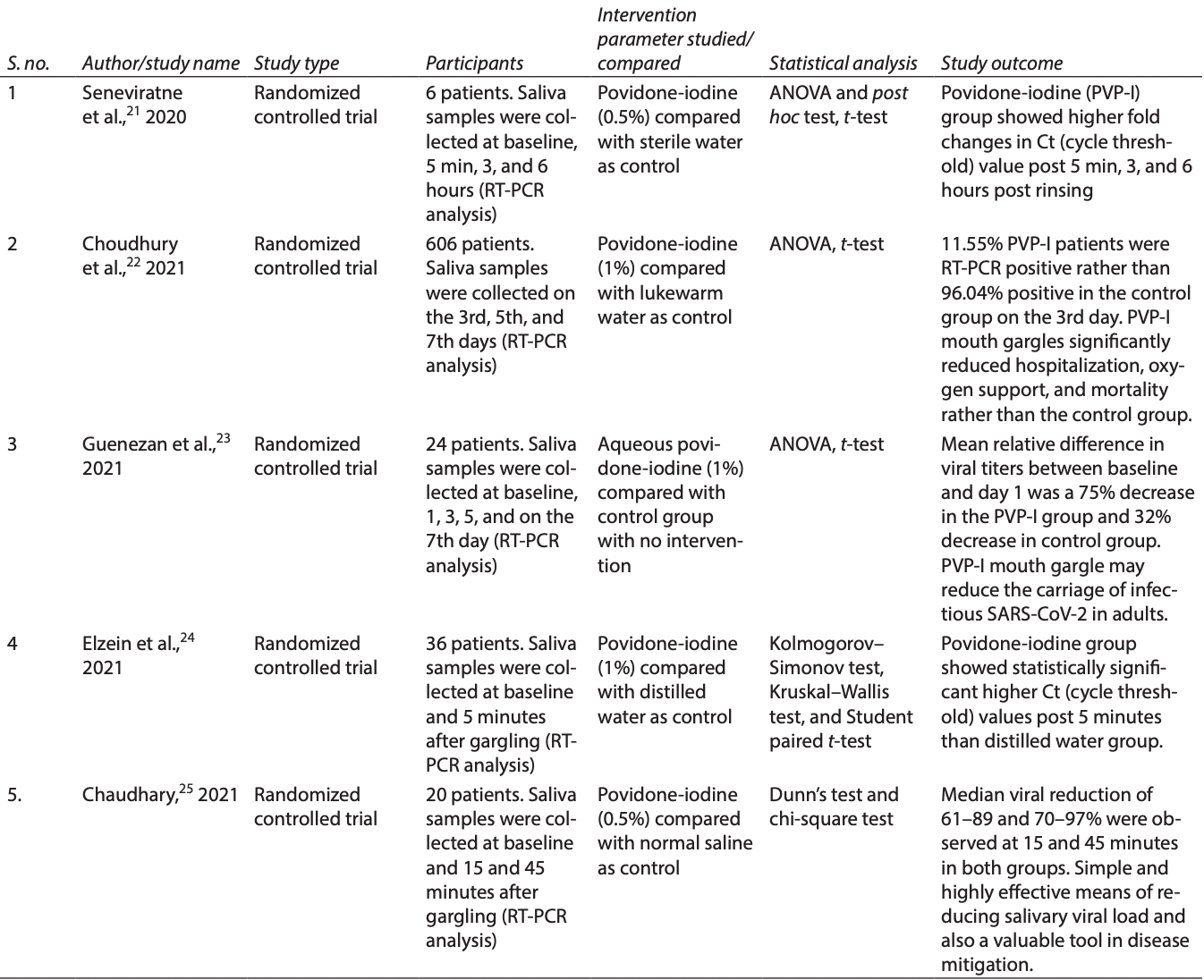
In Vivo Efficacy of Povidone-iodine Mouth Gargles in Reducing Salivary Viral Load in COVID-19 Patients: A Systematic Review
et al., World Journal of Dentistry, doi:10.5005/jp-journals-10015-1868, Nov 2021
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Systematic review of the use of povidone-iodine gargles for COVID-19, concluding that PVP-I effectively reduces SARS-CoV-2 viral load.
3 meta-analyses show significant improvements with povidone-iodine for viral load1-3 and
viral clearance1.
Currently there are 22 povidone-iodine for COVID-19 studies, showing 72% lower mortality [8‑92%], 85% lower hospitalization [73‑91%], and 45% fewer cases [20‑62%].
1.
Hasan et al., Effects of Chlorhexidine and Povidone-Iodine on the SARS-CoV-2 Load: A Systematic Review and Meta-analysis, European Journal of Dentistry, doi:10.1055/s-0042-1753470.
Sudhakar et al., 24 Nov 2021, peer-reviewed, 5 authors.
In Vivo Efficacy of Povidone-iodine Mouth Gargles in Reducing Salivary Viral Load in COVID-19 Patients: A Systematic Review
World Journal of Dentistry, doi:10.5005/jp-journals-10015-1868
Aim and objective: Based on the published research, this article aims to systematically review the in vivo effectiveness of povidone-iodine (PVP-I) mouth gargles in reducing salivary viral load in COVID-19 patients.
Materials and methods: The inhibitory potential of different variables such as PVP-I, chlorhexidine gluconate (CHX), cetylpyridinium chloride (CPC), saline, and hydrogen peroxide (H 2 O 2 ) were tested against SARS-CoV-2 in recent clinical trials. In this systematic review, appropriate randomized controlled trials (RCTs) for the evidence-based question: "what is the efficacy of PVP-I mouth gargle in reducing salivary viral load in COVID-19 patients?" were searched in Medline/PubMed, Scopus, Science Direct, Embase, Google Scholar, and the Cochrane Library database from January 15, 2020, to June 15, 2021, based on defined inclusion and exclusion criteria. From the selected articles, their references and reviews relevant to our topic were also looked for any missed studies. Results: After a pertinent search for appropriate studies, five in vivo RCTs were selected and others were excluded. All the trials used reverse transcription-polymerase chain reaction (RT-PCR) for mRNA detection and quantitation. Povidone-iodine mouth gargle (0.5-1%) used by COVID-19 patients 4th hourly effectively reduced salivary SARS-CoV-2 viral load, thereby reducing the carriage of infectious virion in adults. Statistically significant increase in Ct values, post 5, 15, and 45 minutes, 3 and 6 hours post-rinsing demonstrated the strong antiviral effect of PVP-I.
Conclusion: In this COVID-19 pandemic, based on the published evidence of a few in vivo RCTs, it can be concluded that 0.5 to 1% PVP-I mouth gargle has the potency to effectively reduce the salivary SARS-CoV-2 viral load. To reinforce the use of PVP-I mouth gargles against SARS-CoV-2, this systematic review emphasizes the necessity for future research that is highly focused, robust, and has consistent techniques and a large sample size. Clinical significance: Research on the efficacy of PVP-I mouth gargle should be framed to focus on the most effective minimal concentration, exposure time, and volume of mouth gargle as well as the SARS-CoV-2 strain. The effect of PVP-I mouth gargles on viral infectivity and their cytotoxic effect on epithelial cells were not distinguished in the studies reviewed. Hence, viral cell culture technique should be employed to establish the potential virucidal activity of PVP-I against SARS-CoV-2. Host immunity against SARS-CoV-2 should also be considered in assessing the effectiveness of mouth gargles.
References
Chai, Hu, Zhang, Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection, Genomics, doi:10.1101/2020.02.03.931766
Challacombe, Kirk-Bayley, Sunkaraneni, Povidone iodine, Br Dent J, doi:10.1038/s41415-020-1589-4
Chan, Kok, Zhu, Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan, Emerg Micro Infect, doi:10.1080/22221751.2020.1719902
Chan, Yuan, Kok, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating personto-person transmission: a study of a family cluster, Lancet, doi:10.1016/S0140-6736(20)30154-9
Chaudhary, Melkonyan, Meethil, Estimating salivary carriage of SARS-CoV2 in non-symptomatic individuals and efficacy of mouthwash in reducing viral load: a randomized controlled trial, J Am Dent Assoc
Choudhury, Shabnam, Ahsan, Effect of 1% povidone iodine mouthwash/gargle, nasal and eye drop in COVID-19 patient, Bioresearch Communications, doi:10.3329/brc.v7i1.54245n
Elzein, Sater, Fakhreddine, In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial, J Evid Based Dent Pract, doi:10.1016/j.jebdp.2021.101584
Frank, Capriotti, Brown, Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era, Ear Nose Throat J, doi:10.1177/0145561320932318
Guenezan, Garcia, Strasters, Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.5490
Kanagalingam, Feliciano, Hah, Practical use of povidoneiodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections, Int J Clin Pract, doi:10.1111/ijcp.12707
Kariwa, Fujii, Takashima, Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents, Dermatology, doi:10.1159/000089211
Kawana, Kitamura, Nakagomi, Inactivation of human viruses by povidone-iodine in comparison with other antiseptics, Dermatology, doi:10.1159/000246027
Lamas, Dios, Rodríguez, Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests, Oral Dis, doi:10.1111/odi.13526
Ramalingam, Cai, Wong, Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels, Sci Rep, doi:10.1038/s41598-018-31936-y
Ross, Charles, Dills, Long-term effects of Listerine antiseptic on dental plaque and gingivitis, J Clin Dent
Seneviratne, Balan, Ko, Efficacy of commercial mouthrinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection, doi:10.1007/s15010-020-01563-9
Shankar, Saha, Jamir, Protection at portal of entry (PPE) with povidone iodine for COVID-19, Int J Med Pub Health, doi:10.5530/ijmedph.2020.4.36n
Sneader, Drug discovery: a history, doi:10.1002/0470015535
Sriwilaijaroen, Wilairat, Hiramatsu, Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities, Virol J, doi:10.1186/1743-422X-6-124
Sungnak, Huang, Bécavin, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
To, Tsang, Leung, Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30196-1
Wutzler, Sauerbrei, Klöcking, Virucidal activity and cytotoxicity of the liposomal formulation of povidone-iodine, Antiv Res, doi:10.1016/S0166-3542(01)00213-3
Xu, Chen, Wang, Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission, Sci China Life Sci, doi:10.1007/s11427-020-1637-5
Xu, Cui, Duan, Saliva: potential diagnostic value and transmission of 2019-nCoV, Int J Oral Sci, doi:10.1038/s41368-020-0080-z
Xu, Wang, Hoskin, Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro, Pathogens, doi:10.3390/pathogens10030272
Xu, Zhong, Deng, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int J Oral Sci, doi:10.1038/s41368-020-0074-x
Zhang, Kang, Gong, Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process, Gut, doi:10.1136/gutjnl-2020-320953
Zhao, Zhao, Wang, Single-Cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2, Am J Respir Crit Care Med, doi:10.1164/rccm.202001-0179LE
Zhou, Yang, Wang, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zou, Chen, Zou, Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection, Front Med, doi:10.1007/s11684-020-0754-0
DOI record:
{
"DOI": "10.5005/jp-journals-10015-1868",
"ISSN": [
"0976-6006",
"0976-6014"
],
"URL": "http://dx.doi.org/10.5005/jp-journals-10015-1868",
"alternative-id": [
"10.5005/jp-journals-10015-1868"
],
"author": [
{
"affiliation": [],
"family": "Balasubramanian",
"given": "Balaguhan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Venkatachalapathy",
"given": "Sudhakar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Natarajan",
"given": "Kirthika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aiyathurai",
"given": "Mathan M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sudhakar",
"given": "TS Vinodhini",
"sequence": "additional"
}
],
"container-title": [
"World Journal of Dentistry"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
24
]
],
"date-time": "2021-11-24T13:15:56Z",
"timestamp": 1637759756000
},
"deposited": {
"date-parts": [
[
2021,
11,
24
]
],
"date-time": "2021-11-24T13:16:14Z",
"timestamp": 1637759774000
},
"indexed": {
"date-parts": [
[
2021,
12,
9
]
],
"date-time": "2021-12-09T15:28:58Z",
"timestamp": 1639063738988
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0976-6006"
},
{
"type": "electronic",
"value": "0976-6014"
}
],
"issue": "6",
"issued": {
"date-parts": [
[
2021,
11,
24
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2021
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.wjoud.com/doi/pdf/10.5005/jp-journals-10015-1868",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2914",
"original-title": [],
"page": "504-509",
"prefix": "10.5005",
"published": {
"date-parts": [
[
2021,
11,
24
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
24
]
]
},
"published-print": {
"date-parts": [
[
2021,
11,
24
]
]
},
"publisher": "Jaypee Brothers Medical Publishing",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"doi-asserted-by": "crossref",
"key": "ref=1",
"unstructured": "1. Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395(10223):514–523. DOI: 10.1016/S0140-6736(20)30154-9."
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "crossref",
"key": "ref=2",
"unstructured": "2. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579(7798):270–273. DOI: 10.1038/s41586-020-2012-7."
},
{
"DOI": "10.1080/22221751.2020.1719902",
"doi-asserted-by": "crossref",
"key": "ref=3",
"unstructured": "3. Chan JF-W, Kok K-H, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Micro Infect 2020;9(1):221–236. DOI: 10.1080/22221751.2020.1719902."
},
{
"DOI": "10.1007/s11427-020-1637-5",
"doi-asserted-by": "crossref",
"key": "ref=4",
"unstructured": "4. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63(3):457–460. DOI: 10.1007/s11427-020-1637-5."
},
{
"DOI": "10.1007/s11684-020-0754-0",
"doi-asserted-by": "crossref",
"key": "ref=5",
"unstructured": "5. Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020;14(2):185–192. DOI: 10.1007/s11684-020-0754-0."
},
{
"DOI": "10.1164/rccm.202001-0179LE",
"doi-asserted-by": "crossref",
"key": "ref=6",
"unstructured": "6. Zhao Y, Zhao Z, Wang Y, et al. Single-Cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 2020;202(5):756–759. DOI: 10.1164/rccm.202001-0179LE."
},
{
"DOI": "10.1136/gutjnl-2020-320953",
"doi-asserted-by": "crossref",
"key": "ref=7",
"unstructured": "7. Zhang H, Kang Z, Gong H, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020;69(8):1010–1018. DOI: 10.1136/gutjnl-2020-320953."
},
{
"DOI": "10.1038/s41591-020-0868-6",
"doi-asserted-by": "crossref",
"key": "ref=8",
"unstructured": "8. Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26(5):681–687. DOI: 10.1038/s41591-020-0868-6."
},
{
"DOI": "10.1038/s41368-020-0074-x",
"doi-asserted-by": "crossref",
"key": "ref=9",
"unstructured": "9. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020;12(1):8. DOI: 10.1038/s41368-020-0074-x."
},
{
"DOI": "10.1101/2020.02.03.931766",
"doi-asserted-by": "crossref",
"key": "ref=10",
"unstructured": "10. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Genomics 2020. DOI: 10.1101/2020.02.03.931766."
},
{
"key": "ref=11",
"unstructured": "11. WHO Report of the WHO-China Joint Mission on coronavirus disease 2019 (COVID-19) Feb 28, 2020. https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19). n.d."
},
{
"DOI": "10.1038/s41368-020-0080-z",
"doi-asserted-by": "crossref",
"key": "ref=12",
"unstructured": "12. Xu R, Cui B, Duan X, et al. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci 2020;12(1):11. DOI: 10.1038/s41368-020-0080-z."
},
{
"DOI": "10.1002/0470015535",
"doi-asserted-by": "crossref",
"key": "ref=13",
"unstructured": "13. Sneader W. Drug discovery: a history. 1st ed., Wiley; 2005. DOI: 10.1002/0470015535."
},
{
"DOI": "10.1016/S0166-3542(01)00213-3",
"doi-asserted-by": "crossref",
"key": "ref=14",
"unstructured": "14. Wutzler P, Sauerbrei A, Klöcking R, et al. Virucidal activity and cytotoxicity of the liposomal formulation of povidone-iodine. Antiv Res 2002;54(2):89–97. DOI: 10.1016/S0166-3542(01)00213-3."
},
{
"DOI": "10.1159/000246027",
"doi-asserted-by": "crossref",
"key": "ref=15",
"unstructured": "15. Kawana R, Kitamura T, Nakagomi O, et al. Inactivation of human viruses by povidone-iodine in comparison with other antiseptics. Dermatology 1997;195(2):29–35. DOI: 10.1159/000246027."
},
{
"DOI": "10.1159/000089211",
"doi-asserted-by": "crossref",
"key": "ref=16",
"unstructured": "16. Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 2006;212(4):119–123. DOI: 10.1159/000089211."
},
{
"DOI": "10.1111/ijcp.12707",
"doi-asserted-by": "crossref",
"key": "ref=17",
"unstructured": "17. Kanagalingam J, Feliciano R, Hah JH, et al. Practical use of povidone-iodine antiseptic in the maintenance of oral health and in the prevention and treatment of common oropharyngeal infections. Int J Clin Pract 2015;69(11):1247–1256. DOI: 10.1111/ijcp.12707."
},
{
"DOI": "10.1186/1743-422X-6-124",
"doi-asserted-by": "crossref",
"key": "ref=18",
"unstructured": "18. Sriwilaijaroen N, Wilairat P, Hiramatsu H, et al. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: its effects on hemagglutination and sialidase activities. Virol J 2009;6(1):124. DOI: 10.1186/1743-422X-6-124."
},
{
"DOI": "10.1038/s41415-020-1589-4",
"doi-asserted-by": "crossref",
"key": "ref=19",
"unstructured": "19. Challacombe SJ, Kirk-Bayley J, Sunkaraneni VS, et al. Povidone iodine. Br Dent J 2020;228(9):656–657. DOI: 10.1038/s41415-020-1589-4."
},
{
"DOI": "10.5530/ijmedph.2020.4.36",
"doi-asserted-by": "crossref",
"key": "ref=20",
"unstructured": "20. Shankar S, Saha A, Jamir L, et al. Protection at portal of entry (PPE) with povidone iodine for COVID-19. Int J Med Pub Health 2020;10(4):166–168. DOI: 10.5530/ijmedph.2020.4.36n.d."
},
{
"DOI": "10.1007/s15010-020-01563-9",
"doi-asserted-by": "crossref",
"key": "ref=21",
"unstructured": "21. Seneviratne CJ, Balan P, Ko KKK, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection 2021;49(2):305–311. DOI: 10.1007/s15010-020-01563-9."
},
{
"DOI": "10.3329/brc.v7i1.54245",
"doi-asserted-by": "crossref",
"key": "ref=22",
"unstructured": "22. Choudhury MIM, Shabnam N, Ahsan T, et al. Effect of 1% povidone iodine mouthwash/gargle, nasal and eye drop in COVID-19 patient. Bioresearch Communications 2021;7(1):919–923. DOI: 10.3329/brc.v7i1.54245n.d."
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"doi-asserted-by": "crossref",
"key": "ref=23",
"unstructured": "23. Guenezan J, Garcia M, Strasters D, et al. Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2021;147(4):400. DOI: 10.1001/jamaoto.2020.5490."
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"doi-asserted-by": "crossref",
"key": "ref=24",
"unstructured": "24. Elzein R, Abdel-Sater F, Fakhreddine S, et al. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J Evid Based Dent Pract 2021;21(3):101584. DOI: 10.1016/j.jebdp.2021.101584."
},
{
"key": "ref=25",
"unstructured": "25. Chaudhary PP, Melkonyan A, Meethil A, et al. Estimating salivary carriage of SARS-CoV2 in non-symptomatic individuals and efficacy of mouthwash in reducing viral load: a randomized controlled trial. J Am Dent Assoc 2021. S000281772100355X 10.1016/j.adaj.2021. 05.021."
},
{
"key": "ref=26",
"unstructured": "26. Managing COVID-19 Guidelines. Available online at: https://www.ada.org.au/Campaign/COVID-19/Guide-to-Managing-COVID-19/ADA-Managing COVID-19-Guide-v-2.aspx (accessed June 19, 2021). n.d."
},
{
"key": "ref=27",
"unstructured": "27. Indian Dental Association. Protocol–COVID-19. New Delhi: Indian Dental Association; 2020. p. 26."
},
{
"DOI": "10.1111/odi.13526",
"doi-asserted-by": "crossref",
"key": "ref=28",
"unstructured": "28. Martínez Lamas L, Diz Dios P, Pérez Rodríguez MT, et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis 2020. DOI: 10.1111/odi.13526."
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"doi-asserted-by": "crossref",
"key": "ref=29",
"unstructured": "29. To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20(5):565–574. DOI: 10.1016/S1473-3099(20)30196-1."
},
{
"DOI": "10.1038/s41598-018-31936-y",
"doi-asserted-by": "crossref",
"key": "ref=30",
"unstructured": "30. Ramalingam S, Cai B, Wong J, et al. Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels. Sci Rep 2018;8(1):13630. DOI: 10.1038/s41598-018-31936-y."
},
{
"key": "ref=31",
"unstructured": "31. Ross NM, Charles CH, Dills SS. Long-term effects of Listerine antiseptic on dental plaque and gingivitis. J Clin Dent 1989;1(4):92–95. n.d."
},
{
"DOI": "10.3390/pathogens10030272",
"doi-asserted-by": "crossref",
"key": "ref=32",
"unstructured": "32. Xu C, Wang A, Hoskin ER, et al. Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro. Pathogens 2021;10(3):272. DOI: 10.3390/pathogens10030272."
},
{
"DOI": "10.1177/0145561320932318",
"doi-asserted-by": "crossref",
"key": "ref=33",
"unstructured": "33. Frank S, Capriotti J, Brown SM, et al. Povidone-iodine use in sinonasal and oral cavities: a review of safety in the COVID-19 era. Ear Nose Throat J 2020;99(9):586–593. DOI: 10.1177/0145561320932318."
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"General Dentistry"
],
"subtitle": [],
"title": [
"In Vivo Efficacy of Povidone-iodine Mouth Gargles in Reducing Salivary Viral Load in COVID-19 Patients: A Systematic Review"
],
"type": "journal-article",
"volume": "12"
}
