Long and Short-term Metformin Consumption as a Potential Therapy to Prevent Complications of COVID-19
et al., Advanced Pharmaceutical Bulletin, doi:10.34172/apb.2023.066, IRCT20160310026998N10, Jul 2022
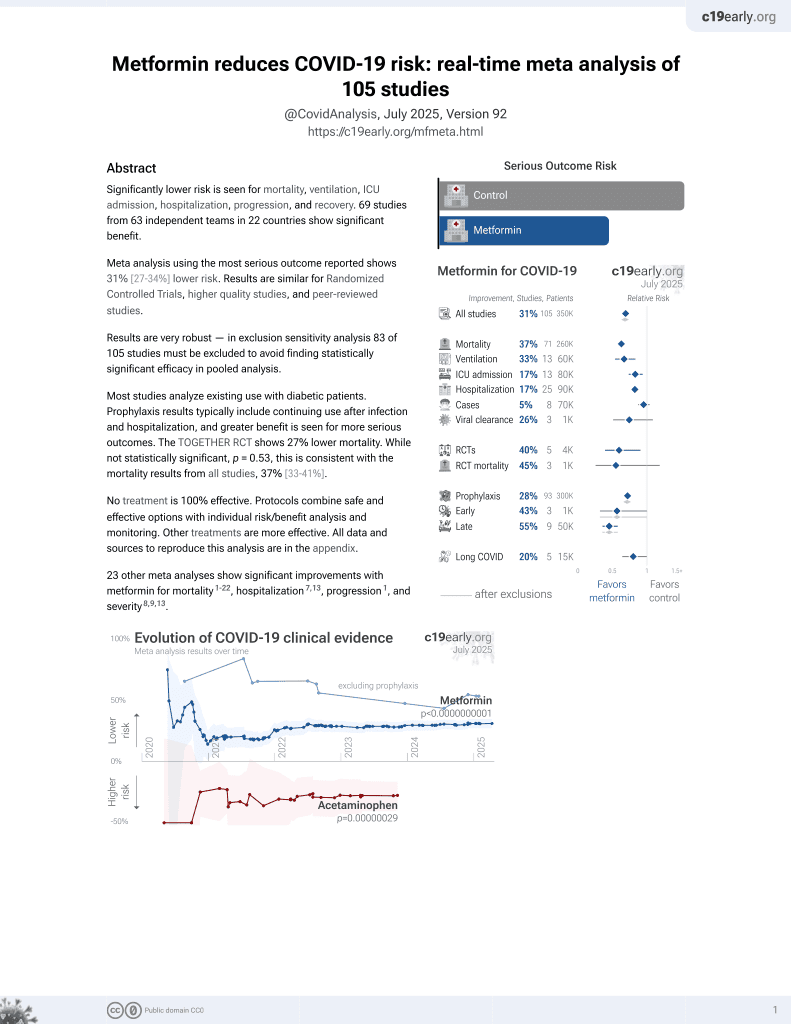
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
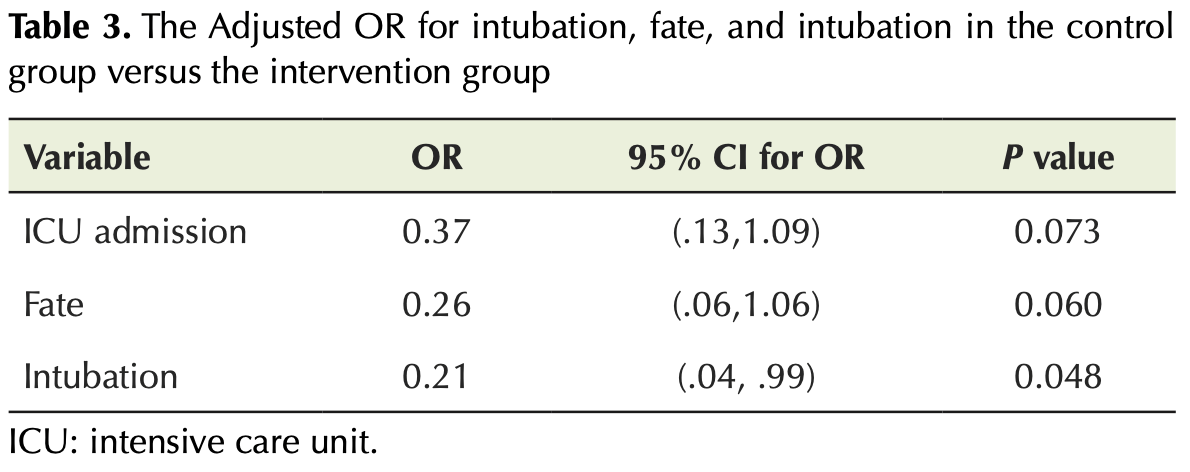
RCT 189 hospitalized patients showing lower mortality, ICU admission, and intubation with metformin, statistically significant only for intubation. Treatment patients may have also taken metformin prior to admission. Authors note that patients receiving metformin prior to the study were not matched, and diabetes and hyperlipidemia differed between groups.
|
risk of death, 74.0% lower, OR 0.26, p = 0.06, treatment 85, control 104, RR approximated with OR.
|
|
risk of mechanical ventilation, 79.0% lower, OR 0.21, p = 0.048, treatment 85, control 104, RR approximated with OR.
|
|
risk of ICU admission, 63.0% lower, OR 0.37, p = 0.07, treatment 85, control 104, RR approximated with OR.
|
|
hospitalization time, 5.0% lower, relative time 0.95, p = 0.52, treatment 85, control 104.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shaseb et al., 2 Jul 2022, Randomized Controlled Trial, Iran, peer-reviewed, 26 authors, study period 20 March, 2020 - 5 April, 2020, trial IRCT20160310026998N10.
Contact: ghaffarys@tbzmed.ac.ir.
Long and Short-term Metformin Consumption as a Potential Therapy to Prevent Complications of COVID-19
Advanced Pharmaceutical Bulletin, doi:10.34172/apb.2023.066
The aim of the study is to evaluate the effect of metformin in complication improvement of hospitalized patients with COVID-19. Methods: This was a randomized clinical trial that involved 189 patients with confirmed COVID-19 infection. Patients in the intervention group received metformin-500 mg twice daily. Patients who received metformin before admission were excluded from the control group. Patients who were discharged before taking at least 2000 mg of metformin were excluded from the study. Primary outcomes were vital signs, need for ICU admission, need for intubation, and mortality. Results: Data showed that patients with diabetes with previous metformin in their regimen had lower percentages of ICU admission and death in comparison with patients without diabetes (11.3% vs. 26.1% (P = 0.014) and 4.9% vs. 23.9% (P ≤ 0.001), respectively). Admission time characteristics were the same for both groups except for diabetes and hyperlipidemia, which were significantly different between the two groups. Observations of naproxen consumption on endpoints, duration of hospitalization, and the levels of spO 2 did not show any significant differences between the intervention and the control group. The adjusted OR for intubation in the intervention group versus the control group was 0.21 [95% CI, 0.04-0.99 (P = 0.047)].
Conclusion: In this trial, metformin consumption had no effect on mortality and ICU admission rates in non-diabetic patients. However, metformin improved COVID-19 complications in diabetic patients who had been receiving metformin prior to COVID-19 infection, and it significantly lowered the intubation rates.
Authors' Contribution
Competing Interests The authors declare no financial or non-financial conflict of interests.
Ethical Approval Full informed consent was obtained from the participants. The study was conducted under the Research Ethics Committee (REC) of Tabriz University of Medical sciences guidelines of good clinical practice under the authorization of the Ministry of Health and Medical Education and the Helsinki Declaration. The presented data are part of the preliminary results of a clinical trial that is registered with the Iranian Registry of Clinical Trials, number IRCT20160310026998N10 and the ethics code of IR.TBZMED. REC.1398.1309.
References
Barron, Bakhai, Kar, Weaver, Bradley et al., Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study, Lancet Diabetes Endocrinol, doi:10.1016/s2213-8587(20)30272-2
Bramante, Buse, Tamaritz, Palacio, Cohen et al., Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity, J Med Virol, doi:10.1002/jmv.26873
Channappanavar, Perlman, Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Semin Immunopathol, doi:10.1007/s00281-017-0629-x
El-Arabey, Abdalla, Eltayb, Metformin, ongoing journey with superdrug revolution, Adv Pharm Bull, doi:10.15171/apb.2019.001
Fadini, Morieri, Longato, Avogaro, Prevalence and impact of diabetes among people infected with SARS-CoV-2, J Endocrinol Invest, doi:10.1007/s40618-020-01236-2
Ferrara, Santilli, 'aiuto, Vitiello, Clinical pharmacology aspects in patients treated with TNF inhibitors during SARS-Cov-2 pandemic, Adv Pharm Bull, doi:10.34172/apb.2021.045
Gupta-Wright, Macleod, Barrett, Filson, Corrah et al., False-negative RT-PCR for COVID-19 and a diagnostic risk score: a retrospective cohort study among patients admitted to hospital, BMJ Open, doi:10.1136/bmjopen-2020-047110
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/s0140-6736(20)30183-5
Jiang, Chen, Liu, Yin, Yang et al., Association of metformin with mortality or ARDS in patients with COVID-19 and type 2 diabetes: a retrospective cohort study, Diabetes Res Clin Pract, doi:10.1016/j.diabres.2020.108619
Kajiwara, Kusaka, Kimura, Yamaguchi, Nanjo et al., Metformin mediates protection against Legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species, J Immunol, doi:10.4049/jimmunol.1700474
Kow, Hasan, Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: a meta-analysis, J Med Virol, doi:10.1002/jmv.26498
Lalau, Al-Salameh, Hadjadj, Goronflot, Wiernsperger et al., Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19, Diabetes Metab, doi:10.1016/j.diabet.2020.101216
Li, Fan, Lai, Han, Li et al., Coronavirus infections and immune responses, J Med Virol, doi:10.1002/jmv.25685
Liang, Ding, Li, Wang, Kan et al., Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies, Crit Care, doi:10.1186/s13054-019-2346-4
Oh, Song, Metformin use and risk of COVID-19 among patients with type II diabetes mellitus: an NHIS-COVID-19 database cohort study, Acta Diabetol, doi:10.1007/s00592-020-01666-7
Rajeshkumar, Yabuuchi, Pai, Maitra, Hidalgo et al., Fatal toxicity of chloroquine or hydroxychloroquine with metformin in mice, bioRxiv, doi:10.1101/2020.03.31.018556v1
Salehi-Pourmehr, Pourfathi, Tarzamni, Ghojazadeh, Naghili et al., Diagnostic value of chest CT in Iranian patients with suspected COVID-19, Caspian J Intern Med, doi:10.22088/cjim.11.0.527
Tian, Jiang, Yao, Nicholson, Li et al., Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis, J Med Virol, doi:10.1002/jmv.26050
Tseng, Metformin decreases risk of tuberculosis infection in type 2 diabetes patients, J Clin Med, doi:10.3390/jcm7090264
Wargny, Potier, Gourdy, Pichelin, Amadou et al., Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study, Diabetologia, doi:10.1007/s00125-020-05351-w
DOI record:
{
"DOI": "10.34172/apb.2023.066",
"ISSN": [
"2228-5881",
"2251-7308"
],
"URL": "http://dx.doi.org/10.34172/apb.2023.066",
"abstract": "<jats:p>Purpose: The aim of the study is to evaluate the effect of metformin in complication improvement of hospitalized patients with COVID-19. Methods: This was a randomized clinical trial that involved 189 patients with confirmed COVID-19 infection. Patients in the intervention group received metformin-500 mg twice daily. Patients who received metformin before admission were excluded from the control group. Patients who were discharged before taking at least 2000 mg of metformin were excluded from the study. Primary outcomes were vital signs, need for ICU admission, need for intubation, and mortality. Results: Data showed that patients with diabetes with previous metformin in their regimen had lower percentages of ICU admission and death in comparison with patients without diabetes (11.3% vs. 26.1% (P=0.014) and 4.9% vs. 23.9% (P≤0.001), respectively). Admission time characteristics were the same for both groups except for diabetes and hyperlipidemia, which were significantly different between the two groups. Observations of naproxen consumption on endpoints, duration of hospitalization, and the levels of spO2 did not show any significant differences between the intervention and the control group. The adjusted OR for intubation in the intervention group versus the control group was 0.21 [95% CI, 0.04-0.99 (P=0.047)]. Conclusion: In this trial, metformin consumption had no effect on mortality and ICU admission rates in non-diabetic patients. However, metformin improved COVID-19 complications in diabetic patients who had been receiving metformin prior to COVID-19 infection, and it significantly lowered the intubation rates. </jats:p>",
"assertion": [
{
"label": "Journal Owner",
"name": "journal_owner",
"value": "Tabriz University of Medical Sciences"
},
{
"label": "Journal Publisher",
"name": "journal_publisher",
"value": "Tabriz University of Medical Sciences"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2021-12-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2022-07-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2022-07-02"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7931-4632",
"affiliation": [
{
"name": "Department of Pharmacotherapy, Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
},
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"authenticated-orcid": true,
"family": "Shaseb",
"given": "Elnaz",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1336-6039",
"affiliation": [
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
},
{
"name": "Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"authenticated-orcid": true,
"family": "Ghaffary",
"given": "Saba",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Garjani",
"given": "Alireza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Tehran University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Zoghi",
"given": "Elnaz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Maleki Dizaji",
"given": "Nasrin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Soltani",
"given": "Somaieh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Road Traffic Injury Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Sarbakhsh",
"given": "Parvin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liver and Gastrointestinal Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Somi",
"given": "Mohammad Hossein",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, Tehran University of Medical Sciences, Tehran, Iran."
}
],
"family": "Valizadeh",
"given": "Parya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tuberculosis and Lung Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Taghizadieh",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Liver and Gastrointestinal Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Faghihdinevari",
"given": "Masood",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Varshochi",
"given": "Mojtaba",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Naghily",
"given": "Behrooz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Bayatmakoo",
"given": "Zhinous",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Saleh",
"given": "Parviz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Taghizadeh",
"given": "Sepehr",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Haghdoost",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Owaysi",
"given": "Hamid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Ravanbakhsh Ghavghani",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology, Medical Radiation Sciences Research Group, Imam Reza Hospital, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Tarzamni",
"given": "Mohammad Kazem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology, Medical Radiation Sciences Research Group, Imam Reza Hospital, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Moradi",
"given": "Rojin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Javan Ali Azar",
"given": "Fateme",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacotherapy, Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Shabestari Khiabani",
"given": "Saeid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imam Reza Hospital, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Ghazanchaei",
"given": "Ardavan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Hamedani",
"given": "Sana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran."
}
],
"family": "Hatefi",
"given": "Shahabeddin",
"sequence": "additional"
}
],
"container-title": "Advanced Pharmaceutical Bulletin",
"container-title-short": "Adv Pharm Bull",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"apb.tbzmed.ac.ir"
]
},
"created": {
"date-parts": [
[
2022,
8,
28
]
],
"date-time": "2022-08-28T09:10:05Z",
"timestamp": 1661677805000
},
"deposited": {
"date-parts": [
[
2023,
7,
22
]
],
"date-time": "2023-07-22T06:11:13Z",
"timestamp": 1690006273000
},
"indexed": {
"date-parts": [
[
2023,
7,
23
]
],
"date-time": "2023-07-23T04:12:49Z",
"timestamp": 1690085569369
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
7,
2
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2023,
7,
10
]
]
},
"published-print": {
"date-parts": [
[
2023,
7,
10
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://apb.tbzmed.ac.ir/PDF/apb-13-621.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://apb.tbzmed.ac.ir/PDF/apb-13-621.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "20123",
"original-title": [],
"page": "621-626",
"prefix": "10.34172",
"published": {
"date-parts": [
[
2022,
7,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
2
]
]
},
"published-print": {
"date-parts": [
[
2023,
7,
10
]
]
},
"publisher": "Maad Rayan Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://apb.tbzmed.ac.ir/Article/apb-33452"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Long and Short-term Metformin Consumption as a Potential Therapy to Prevent Complications of COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.34172/crossmark_policy",
"volume": "13"
}