Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada
et al., medRxiv, doi:10.1101/2022.11.03.22281881, Nov 2022
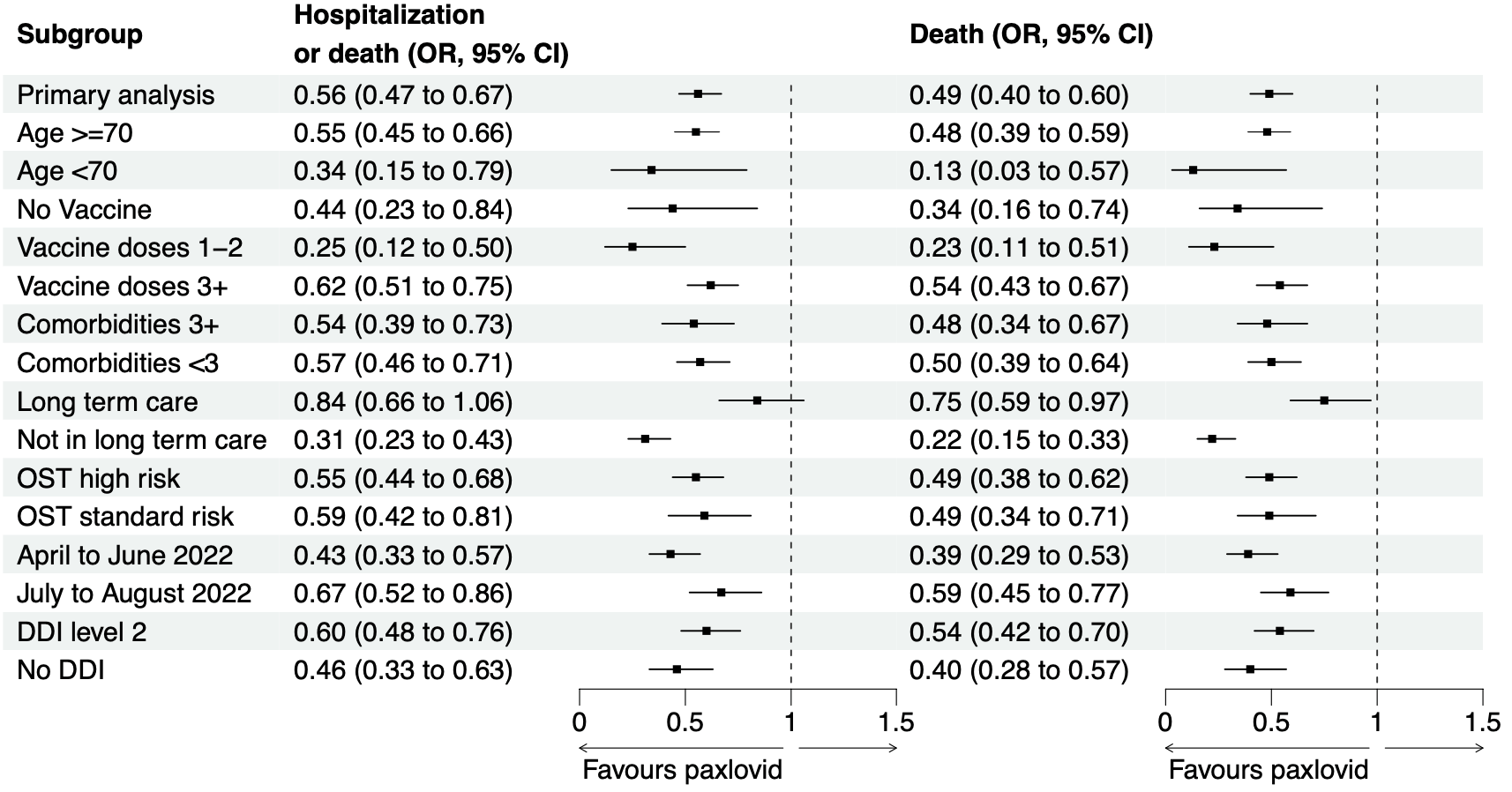
Retrospective 177,545 patients in Canada, 8,876 treated with paxlovid, showing lower mortality and hospitalization with treatment, and declining efficacy over the two time periods analyzed.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
|
risk of death, 50.2% lower, RR 0.50, p < 0.001, treatment 142 of 8,876 (1.6%), control 5,566 of 168,669 (3.3%), NNT 59, odds ratio converted to relative risk, propensity score weighting.
|
|
risk of death/hospitalization, 43.1% lower, RR 0.57, p < 0.001, treatment 186 of 8,876 (2.1%), control 6,241 of 168,669 (3.7%), NNT 62, odds ratio converted to relative risk, propensity score weighting, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Schwartz et al., 5 Nov 2022, retrospective, Canada, preprint, mean age 74.0, 10 authors, study period 4 April, 2022 - 31 August, 2022.
Contact: kevin.schwartz@oahpp.ca.
Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada
doi:10.1101/2022.11.03.22281881
Background: Our objective was to evaluate the real world effectiveness of nirmatrelvir/ritonavir to prevent severe COVID-19 while Omicron and its subvariants predominate. Methods: We conducted a population based cohort study in Ontario, Canada including all residents >17 years of age who tested positive for SARS-CoV-2 by PCR between 4 April and 31 August 2022. We compared nirmatrelvir/ritonavir treated patients to unexposed patients and measured the primary outcome of hospitalization or death from COVID-19, and a secondary outcome of death 1-30 days. We used weighted logistic regression to calculate weighted odds ratios (wOR) with 95% confidence intervals (CIs) using inverse probability of treatment weighting (IPTW) to control for confounding. Results: The final cohort included 177,545 patients with 8,876 (5.0%) exposed and 168,669 (95.0%) unexposed individuals. The groups were well balanced with respect to demographic and clinical characteristics after applying stabilized IPTW. Hospitalization or death within 30 days was lower in the nirmatrelvir/ritonavir treated group compared to unexposed individuals (2.1% vs 3.7%, wOR 0.56; 95%CI, 0.47-0.67). In the secondary analysis, the relative odds of death was also significantly reduced (1.6% vs 3.3%, wOR 0.49; 95%CI, 0.39-0.62). The number needed to treat to prevent one case of severe COVID-19 was 62 (95%CI 43 to 80). Findings were similar across strata of age, DDIs, vaccination status, and comorbidities. Interpretation: Nirmatrelvir/ritonavir was associated with significantly reduced risk of hospitalization and death from COVID-19 in this observational study, supporting ongoing use of this therapeutic to treat patients with mild COVID-19 at risk for severe disease. .
References
Agrawal, Bedston, Mccowan, Oke, Patterson et al., Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales, Lancet
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med
Austin, Stuart, Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies, Stat Med
Chung, He, Nasreen, Sundaram, Buchan et al., Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study, BMJ
Cole, Hernán, Constructing inverse probability weights for marginal structural models, Am J Epidemiol
Ganatra, Dani, Ahmad, Kumar, Shah et al., Oral Nirmatrelvir and Ritonavir in Nonhospitalized Vaccinated Patients With Coronavirus Disease 2019 (COVID-19), Clin Infect Dis
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Heilmann, Costacurta, Moghadasi, Ye, Pavan et al., SARS-CoV-2 3CLpro mutations selected in a VSV-based system confer resistance to nirmatrelvir, ensitrelvir, and GC376, Sci Transl Med
Komorowski, Evidence-based recommendations on the use of nirmatrelvir/ritonavir (Paxlovid) for adults in Ontario
Levy, 'brien, Sellors, Grootendorst, Willison, Coding accuracy of administrative drug claims in the Ontario Drug Benefit database, Can J Clin Pharmacol
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Prescribing Nirmatrelvir-Ritonavir: How to Recognize and Manage Drug-Drug Interactions, Ann Intern Med
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clin Infect Dis
Nirmatrelvir/Ritonavir, (Paxlovid): What Prescribers and Pharmacists Need to Know Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group on behalf of the Ontario COVID-19, Science Advisory Table and University of Waterloo School of Pharmacy Version, doi:10.47326/ocsat.2022.03.58.1.0
Suissa, Immortal time bias in pharmaco-epidemiology, Am J Epidemiol
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med
Ulloa, Buchan, Daneman, Brown, Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada, JAMA
Xu, Ross, Raebel, Shetterly, Blanchette et al., Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals, Value Health
Yip, Lui, Lai, Wong, Tse et al., Impact of the use of oral antiviral agents on the risk of hospitalization in community COVID-19 patients, Clin Infect Dis [Internet
Zheng, Ma, Wang, Chen, Zhou et al., Efficacy and safety of Paxlovid for COVID-19:a meta-analysis, J Infect
DOI record:
{
"DOI": "10.1101/2022.11.03.22281881",
"URL": "http://dx.doi.org/10.1101/2022.11.03.22281881",
"abstract": "<jats:p>Background: Our objective was to evaluate the real world effectiveness of nirmatrelvir/ritonavir to prevent severe COVID-19 while Omicron and its subvariants predominate. Methods: We conducted a population based cohort study in Ontario, Canada including all residents >17 years of age who tested positive for SARS-CoV-2 by PCR between 4 April and 31 August 2022. We compared nirmatrelvir/ritonavir treated patients to unexposed patients and measured the primary outcome of hospitalization or death from COVID-19, and a secondary outcome of death 1-30 days. We used weighted logistic regression to calculate weighted odds ratios (wOR) with 95% confidence intervals (CIs) using inverse probability of treatment weighting (IPTW) to control for confounding. Results: The final cohort included 177,545 patients with 8,876 (5.0%) exposed and 168,669 (95.0%) unexposed individuals. The groups were well balanced with respect to demographic and clinical characteristics after applying stabilized IPTW. Hospitalization or death within 30 days was lower in the nirmatrelvir/ritonavir treated group compared to unexposed individuals (2.1% vs 3.7%, wOR 0.56; 95%CI, 0.47-0.67). In the secondary analysis, the relative odds of death was also significantly reduced (1.6% vs 3.3%, wOR 0.49; 95%CI, 0.39-0.62). The number needed to treat to prevent one case of severe COVID-19 was 62 (95%CI 43 to 80). Findings were similar across strata of age, DDIs, vaccination status, and comorbidities. Interpretation: Nirmatrelvir/ritonavir was associated with significantly reduced risk of hospitalization and death from COVID-19 in this observational study, supporting ongoing use of this therapeutic to treat patients with mild COVID-19 at risk for severe disease.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
11,
5
]
]
},
"author": [
{
"affiliation": [],
"family": "Schwartz",
"given": "Kevin L",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tadrous",
"given": "Mina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langford",
"given": "Bradley J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daneman",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leung",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomes",
"given": "Tara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Friedman",
"given": "Lindsay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daley",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Kevin A",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
6
]
],
"date-time": "2022-11-06T02:15:10Z",
"timestamp": 1667700910000
},
"deposited": {
"date-parts": [
[
2022,
11,
6
]
],
"date-time": "2022-11-06T02:15:10Z",
"timestamp": 1667700910000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
11,
6
]
],
"date-time": "2022-11-06T04:53:29Z",
"timestamp": 1667710409073
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
5
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.11.03.22281881",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
11,
5
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
11,
5
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.11.03.22281881"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada",
"type": "posted-content"
}