Effect of Paxlovid treatment during acute COVID-19 on Long COVID onset: An EHR-based target trial emulation from the N3C and RECOVER consortia
et al., PLOS Medicine, doi:10.1371/journal.pmed.1004711, Jan 2024 (preprint)
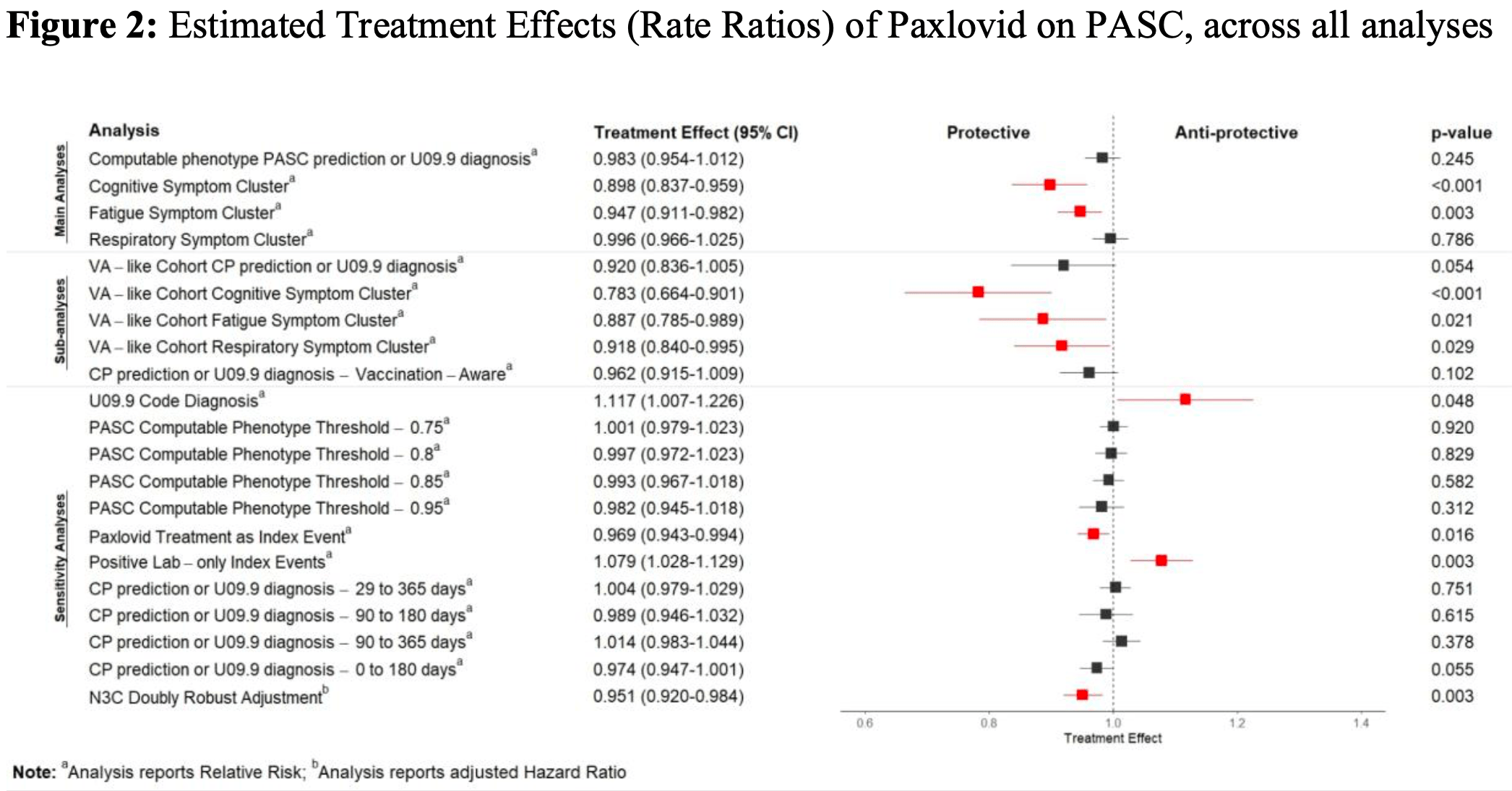
Retrospective 445,378 high-risk outpatients showing no significant difference in post-acute sequelae of COVID-19 (PASC) incidence with paxlovid treatment. Subgroup analysis showed benefits for cognitive and fatigue symptoms. The study used a large, nationally representative sample and a machine learning model to identify PASC cases.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments18.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of long COVID, 1.0% lower, RR 0.99, p = 0.59, adjusted per study.
|
|
cognitive symptom cluster, 9.0% lower, RR 0.91, p = 0.02, adjusted per study.
|
|
fatigue symptom cluster, 6.0% lower, RR 0.94, p = 0.004, adjusted per study.
|
|
respiratory symptom cluster, 1.0% higher, RR 1.01, p = 0.58, adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Preiss et al., 22 Jan 2024, retrospective, USA, peer-reviewed, 18 authors, study period 1 April, 2022 - 28 February, 2023.
Contact: apreiss@rti.org.
Effect of Paxlovid treatment during acute COVID-19 on Long COVID onset: An EHR-based target trial emulation from the N3C and RECOVER consortia
PLOS Medicine, doi:10.1371/journal.pmed.1004711
Background Preventing and treating post-acute sequelae of COVID-19 infection (PASC), commonly known as Long COVID, has become a public health priority. This study tests whether Paxlovid treatment in the acute phase of COVID-19 could help prevent the onset of PASC.
Methods and findings We used electronic health records from the National Clinical Cohort Collaborative to define a cohort of 445,738 patients who had COVID-19 since April 1, 2022, and were eligible for Paxlovid treatment due to risk for progression to severe COVID-19. We used the target trial emulation framework to estimate the effect of Paxlovid treatment on PASC incidence. We emulated a series of six sequential trials: one for each day of a 5-day treatment grace period. For each sequential trial, the treatment group was defined as patients prescribed Paxlovid on the trial start day, and the control group was defined as all patients meeting eligibility criteria who remained untreated on the trial start day. We pooled individual record-level data from the sequential trials for analysis. The follow-up period was 180 days. The primary outcome was overall PASC incidence measured using a computable phenotype. Secondary outcomes were incident cognitive, fatigue, and respiratory symptoms in the post-acute period. We controlled for a wide range of demographic and medical history covariates. Compared to the control group, Paxlovid treatment did not have a significant effect on overall PASC incidence or incident respiratory symptoms. It had a small protective effect against cognitive (relative risk [RR] 0.91; 95% CI [0.84, 0.98]; p = 0.019) and fatigue (RR 0.94; 95% CI [0.90, 0.98]; p = 0.002) symptoms. Finally, we estimated Paxlovid's effect on overall PASC incidence across strata of age, COVID-19 vaccination status, and Charlson Comorbidity Index (CCI) prior to COVID-19. We found small protective effects among patients aged 65 years or more (RR 0.92; 95% CI [0.88, 0.97]; p < 0.001; absolute risk difference [ARD] -0.43%; number needed to treat [NNT] 233) and with a CCI of 3 or 4 (RR 0.83; 95% CI [0.75, 0.92]; p < 0.001; ARD -1.30%; NNT 76). This study's main limitation is that the causal interpretation relies on the assumption that we controlled for all confounding variables.
Conclusions Although some prior observational studies suggested that Paxlovid held promise as a PASC preventive, this study-with a large, nationally sampled cohort; a contemporary study period; and causal inference methodology-found that Paxlovid treatment during acute COVID-19 had no effect on subsequent PASC incidence. Stratified analyses suggest that Paxlovid may have a small protective effect among higher-risk patients, but the NNT is high. In conclusion, we see Paxlovid as unlikely to become a definitive solution for PASC prevention.
Analysis Cumulative
References
Admon, Donnelly, Casey, Janz, Russell et al., Emulating a novel clinical trial using existing observational data. predicting results of the PreVent study, Ann Am Thorac Soc, doi:10.1513/AnnalsATS.201903-241OC
Anschuetz, DU study finds history of TBI likely worsens Long COVID symptoms
Bhatia, Preiss, Xiao, Brannock, Alexander et al., Effect of nirmatrelvir/ritonavir (Paxlovid) on hospitalization among adults with COVID-19: an EHR-based target trial emulation from N3C. medRxiv, doi:10.1101/2023.05.03.23289084
Bose-Brill, Hirabayashi, Pajor, Rao, Mejias et al., Pediatric nirmatrelvir/ritonavir prescribing patterns during the COVID-19 pandemic, medRxiv, doi:10.1101/2022.12.23.22283868
Burden, Long, Collaborators, Wulf Hanson, Abbafati et al., Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021, JAMA, doi:10.1001/jama.2022.18931
Burgner, Crawford, Goeman, Gray, Hsu, COVID-19 in children. II: pathogenesis, disease spectrum and management, J Paediatr Child Health, doi:10.1111/jpc.15811
Cdc, Interim clinical considerations for COVID-19 treatment in outpatients, Centers for Disease Control and Prevention
Cohen, Jaudon, Schurman, Kava, Vogel et al., Impact of extended-course oral nirmatrelvir/ritonavir (Paxlovid) in established Long COVID: case series and research considerations, Res Sq, doi:10.21203/rs.3.rs-3359429/v1
Congdon, Narrowe, Yone, Gunn, Deng et al., Nirmatrelvir/ritonavir and risk of Long COVID symptoms: a retrospective cohort study, doi:10.21203/rs.3.rs-3231786/v1
Consulting, What we heard: engagement report on the working definition for Long COVID
Crosskey, Mcintee, Preiss, Brannock, Yoo et al., Reengineering a machine learning phenotype to adapt to the changing COVID-19 landscape: a study from the N3C and RECOVER consortia, bioRxiv, doi:10.1101/2023.12.08.23299718
Dickerman, García-Albéniz, Logan, Denaxas, Hernán, Avoidable flaws in observational analyses: an application to statins and cancer, Nat Med, doi:10.1038/s41591-019-0597-x
Dryden-Peterson, Kim, Kim, Caniglia, Lennes et al., Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health System : a population-based cohort study, Ann Intern Med, doi:10.7326/M22-2141
Durstenfeld, Peluso, Lin, Peyser, Isasi et al., Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study, J Med Virol, doi:10.1002/jmv.29333
Fang, Li, Yu, Wang, Zhang et al., Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis, Aging, doi:10.18632/aging.103579
Fernández-De-Las-Peñas, Palacios-Ceña, Gómez-Mayordomo, Florencio, Cuadrado et al., Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis, Eur J Intern Med, doi:10.1016/j.ejim.2021.06.009
Gbinigie, Ogburn, Allen, Dorward, Dobson et al., Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community (PANORAMIC): protocol for a randomised, controlled, open-label, adaptive platform trial of community novel antiviral treatment of COVID-19 in people at increased risk of more severe disease, BMJ Open, doi:10.1136/bmjopen-2022-069176
Geng, Bonilla, Hedlin, Jacobson, Tian et al., Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial, JAMA Intern Med, doi:10.1001/jamainternmed.2024.2007
Geng, Bonilla, Shafer, Miglis, Yang, The use of nirmatrelvir-ritonavir in a case of breakthrough Long COVID, Explor Res Hypothesis Med, doi:10.14218/erhm.2022.00045
Granados, Sáez-López, Aljama, Sampol, Cruz et al., Asbestos exposure and severity of COVID-19, Int J Environ Res Public Health, doi:10.3390/ijerph192316305
Gupta, Wang, Hayek, Chan, Mathews et al., Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19, JAMA Intern Med, doi:10.1001/jamainternmed.2020.6252
Haendel, Chute, Bennett, Eichmann, Guinney et al., The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment, J Am Med Inform Assoc, doi:10.1093/jamia/ocaa196
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hernán, Leaf, Target trial emulation: a framework for causal inference from observational data, JAMA, doi:10.1001/jama.2022.21383
Hernán, Robins, Using Big Data to emulate a target trial when a randomized trial is not available, Am J Epidemiol, doi:10.1093/aje/kwv254
Hill, Mehta, Sharma, Mane, Singh et al., Risk factors associated with post-acute sequelae of SARS-CoV-2: an N3C and NIH RECOVER study, BMC Public Health, doi:10.1186/s12889-023-16916-w
Honardoost, Janani, Aghili, Emami, Khamseh, The association between presence of comorbidities and COVID-19 severity: a systematic review and meta-analysis, Cerebrovasc Dis, doi:10.1159/000513288
Imai, King, Stuart, Misunderstandings between experimentalists and observationalists about causal inference, J R Stat Soc Ser A Stat Soc, doi:10.1111/j.1467-985x.2007.00527.x
Ioannou, Berry, Rajeevan, Li, Mutalik et al., Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. veterans : a target trial emulation, Ann Intern Med, doi:10.7326/M23-1394
Iwasaki, Putrino, Why we need a deeper understanding of the pathophysiology of Long COVID, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00053-1
Lin, Fadel, Huang, Milinovich, Sacha et al., Nirmatrelvir or molnupiravir use and severe outcomes from omicron infections, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.35077
Lin, Glynn, Singer, Murphy, Lii et al., Out-of-system care and recording of patient characteristics critical for comparative effectiveness research, Epidemiology, doi:10.1097/EDE.0000000000000794
Malden, Hong, Lewin, Hospitalization and emergency department encounters for COVID-19 after Paxlovid treatment-California, MMWR, doi:10.15585/mmwr.mm7125
Mccarthy, Paxlovid as a potential treatment for Long COVID, Expert Opin Pharmacother, doi:10.1080/14656566.2023.2262387
Morgan, Teal, Reddy, Ford, Ashton, Measurement in Veterans Affairs Health Services Research: veterans as a special population, Health Serv Res, doi:10.1111/j.1475-6773.2005.00448.x
Munblit, Hara, Akrami, Perego, Olliaro et al., Long COVID: aiming for a consensus, Lancet Respir Med, doi:10.1016/S2213-2600(22)00135-7
Munz, Why should veterans diagnosed with mesothelioma seek legal help? Mesothelioma Center-Vital Services for Cancer Patients & Families
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Peluso, Anglin, Durstenfeld, Martin, Kelly et al., Effect of oral nirmatrelvir on Long COVID symptoms: 4 cases and rationale for systematic studies, Pathog Immun, doi:10.20411/pai.v7i1.518
Perez Giraldo, Ali, Kang, Patel, Budhiraja et al., Neurologic manifestations of Long COVID differ based on acute COVID-19 severity, Ann Neurol, doi:10.1002/ana.26649
Petito, García-Albéniz, Logan, Howlader, Mariotto et al., Estimates of overall survival in patients with cancer receiving different treatment regimens: emulating hypothetical target trials in the surveillance, epidemiology, and end results (SEER)-Medicare linked database, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.0452
Pfaff, Girvin, Gabriel, Kostka, Morris et al., Synergies between centralized and federated approaches to data quality: a report from the national COVID cohort collaborative, J Am Med Inform Assoc, doi:10.1093/jamia/ocab217
Sanyaolu, Okorie, Marinkovic, Patidar, Younis et al., Comorbidity and its impact on patients with COVID-19, SN Compr Clin Med, doi:10.1007/s42399-020-00363-4
Schneider, Peltz, Li, Bahorik, Gardner et al., Traumatic brain injury and long-term risk of stroke among US military veterans, Stroke, doi:10.1161/STROKEAHA.123.042360
Soriano, Murthy, Marshall, Relan, Diaz, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00703-9
Stuart, Matching methods for causal inference: a review and a look forward, Stat Sci, doi:10.1214/09-STS313
Van Walraven, Bennett, Forster, Administrative database research infrequently used validated diagnostic or procedural codes, J Clin Epidemiol, doi:10.1016/j.jclinepi.2011.01.001
Veroniki, Seitidis, Tsivgoulis, Katsanos, Mavridis, An introduction to individual participant data meta-analysis, Neurology, doi:10.1212/WNL.0000000000207078
Visvabharathy, Orban, Koralnik, Case report: treatment of Long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence, Front Med, doi:10.3389/fmed.2022.1003103
Wang, Shaw, Mathelier, Kimmel, French, Evaluating risk-prediction models using data from electronic health records, Ann Appl Stat, doi:10.1214/15-AOAS891
Wells, Chagin, Nowacki, Kattan, Strategies for handling missing data in electronic health record derived data, EGEMS, doi:10.13063/2327-9214.1035
Xie, Bowe, Al-Aly, Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status, Nat Commun, doi:10.1038/s41467-021-26513-3
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Intern Med, doi:10.1001/jamainternmed.2023.0743
Yip, Lui, Lai, Wong, Tse et al., Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciac687
DOI record:
{
"DOI": "10.1371/journal.pmed.1004711",
"ISSN": [
"1549-1676"
],
"URL": "http://dx.doi.org/10.1371/journal.pmed.1004711",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Preventing and treating post-acute sequelae of COVID-19 infection (PASC), commonly known as Long COVID, has become a public health priority. This study tests whether Paxlovid treatment in the acute phase of COVID-19 could help prevent the onset of PASC.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>We used electronic health records from the National Clinical Cohort Collaborative to define a cohort of 445,738 patients who had COVID-19 since April 1, 2022, and were eligible for Paxlovid treatment due to risk for progression to severe COVID-19. We used the target trial emulation framework to estimate the effect of Paxlovid treatment on PASC incidence. We emulated a series of six sequential trials: one for each day of a 5-day treatment grace period. For each sequential trial, the treatment group was defined as patients prescribed Paxlovid on the trial start day, and the control group was defined as all patients meeting eligibility criteria who remained untreated on the trial start day. We pooled individual record-level data from the sequential trials for analysis. The follow-up period was 180 days. The primary outcome was overall PASC incidence measured using a computable phenotype. Secondary outcomes were incident cognitive, fatigue, and respiratory symptoms in the post-acute period. We controlled for a wide range of demographic and medical history covariates. Compared to the control group, Paxlovid treatment did not have a significant effect on overall PASC incidence or incident respiratory symptoms. It had a small protective effect against cognitive (relative risk [RR] 0.91; 95% CI [0.84, 0.98]; <jats:italic>p</jats:italic> = 0.019) and fatigue (RR 0.94; 95% CI [0.90, 0.98]; <jats:italic>p</jats:italic> = 0.002) symptoms. Finally, we estimated Paxlovid’s effect on overall PASC incidence across strata of age, COVID-19 vaccination status, and Charlson Comorbidity Index (CCI) prior to COVID-19. We found small protective effects among patients aged 65 years or more (RR 0.92; 95% CI [0.88, 0.97]; <jats:italic>p</jats:italic> < 0.001; absolute risk difference [ARD] −0.43%; number needed to treat [NNT] 233) and with a CCI of 3 or 4 (RR 0.83; 95% CI [0.75, 0.92]; <jats:italic>p</jats:italic> < 0.001; ARD −1.30%; NNT 76). This study’s main limitation is that the causal interpretation relies on the assumption that we controlled for all confounding variables.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusions</jats:title>\n<jats:p>Although some prior observational studies suggested that Paxlovid held promise as a PASC preventive, this study—with a large, nationally sampled cohort; a contemporary study period; and causal inference methodology—found that Paxlovid treatment during acute COVID-19 had no effect on subsequent PASC incidence. Stratified analyses suggest that Paxlovid may have a small protective effect among higher-risk patients, but the NNT is high. In conclusion, we see Paxlovid as unlikely to become a definitive solution for PASC prevention.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "https://orcid.org/0000-0002-7966-527X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Preiss",
"given": "Alexander",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-0063-368X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Bhatia",
"given": "Abhishek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aragon",
"given": "Leyna V.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1124-7928",
"affiliation": [],
"authenticated-orcid": true,
"family": "Baratta",
"given": "John M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baskaran",
"given": "Monika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blancero",
"given": "Frank",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8095-547X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Brannock",
"given": "Michael Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chew",
"given": "Robert F.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9056-2047",
"affiliation": [],
"authenticated-orcid": true,
"family": "Díaz",
"given": "Iván",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fitzgerald",
"given": "Megan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3722-8928",
"affiliation": [],
"authenticated-orcid": true,
"family": "Kelly",
"given": "Elizabeth P.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1845-5620",
"affiliation": [],
"authenticated-orcid": true,
"family": "Zhou",
"given": "Andrea G.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0059-1990",
"affiliation": [],
"authenticated-orcid": true,
"family": "Carton",
"given": "Thomas W.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5437-2545",
"affiliation": [],
"authenticated-orcid": true,
"family": "Chute",
"given": "Christopher G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haendel",
"given": "Melissa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2723-5902",
"affiliation": [],
"authenticated-orcid": true,
"family": "Moffitt",
"given": "Richard",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6840-9756",
"affiliation": [],
"authenticated-orcid": true,
"family": "Pfaff",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of the N3C Consortium and the RECOVER Cohort",
"sequence": "additional"
}
],
"container-title": "PLOS Medicine",
"container-title-short": "PLoS Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosmedicine.org"
]
},
"created": {
"date-parts": [
[
2025,
9,
15
]
],
"date-time": "2025-09-15T17:40:28Z",
"timestamp": 1757958028000
},
"deposited": {
"date-parts": [
[
2025,
9,
18
]
],
"date-time": "2025-09-18T17:55:02Z",
"timestamp": 1758218102000
},
"editor": [
{
"affiliation": [],
"family": "Mody",
"given": "Aaloke",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100000050",
"award": [
"OTA OT2HL161847"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000050",
"id-type": "DOI"
}
],
"name": "National Heart, Lung, and Blood Institute"
}
],
"indexed": {
"date-parts": [
[
2025,
9,
29
]
],
"date-time": "2025-09-29T11:56:17Z",
"timestamp": 1759146977901,
"version": "3.44.0"
},
"is-referenced-by-count": 1,
"issue": "9",
"issued": {
"date-parts": [
[
2025,
9,
15
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2025,
9,
15
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
9,
15
]
],
"date-time": "2025-09-15T00:00:00Z",
"timestamp": 1757894400000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pmed.1004711",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e1004711",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2025,
9,
15
]
]
},
"published-online": {
"date-parts": [
[
2025,
9,
15
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.7326/M22-2141",
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 in a large U.S. Health System : a population-based cohort study",
"author": "S Dryden-Peterson",
"doi-asserted-by": "crossref",
"first-page": "77",
"issue": "1",
"journal-title": "Ann Intern Med",
"key": "pmed.1004711.ref001",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "J Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "pmed.1004711.ref002",
"volume": "386",
"year": "2022"
},
{
"article-title": "Effect of nirmatrelvir/ritonavir (Paxlovid) on hospitalization among adults with COVID-19: an EHR-based target trial emulation from N3C",
"author": "A Bhatia",
"journal-title": "medRxiv",
"key": "pmed.1004711.ref003",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "pmed.1004711.ref004",
"volume": "76",
"year": "2023"
},
{
"article-title": "Hospitalization and emergency department encounters for COVID-19 after Paxlovid treatment—California",
"author": "DE Malden",
"first-page": "830",
"journal-title": "MMWR",
"key": "pmed.1004711.ref005",
"year": "2022"
},
{
"article-title": "Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19)",
"author": "TC-F Yip",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "pmed.1004711.ref006",
"volume": "76",
"year": "2023"
},
{
"article-title": "Nirmatrelvir or molnupiravir use and severe outcomes from omicron infections",
"author": "D-Y Lin",
"issue": "9",
"journal-title": "JAMA Netw Open",
"key": "pmed.1004711.ref007",
"volume": "6",
"year": "2023"
},
{
"article-title": "Effect of oral nirmatrelvir on Long COVID symptoms: 4 cases and rationale for systematic studies",
"author": "MJ Peluso",
"first-page": "95",
"issue": "1",
"journal-title": "Pathog Immun",
"key": "pmed.1004711.ref008",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.14218/ERHM.2022.00045",
"article-title": "The use of nirmatrelvir-ritonavir in a case of breakthrough Long COVID",
"author": "LN Geng",
"doi-asserted-by": "crossref",
"first-page": "394",
"journal-title": "Explor Res Hypothesis Med",
"key": "pmed.1004711.ref009",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2022.1003103",
"article-title": "Case report: treatment of Long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence",
"author": "L Visvabharathy",
"doi-asserted-by": "crossref",
"first-page": "1003103",
"journal-title": "Front Med (Lausanne)",
"key": "pmed.1004711.ref010",
"volume": "9",
"year": "2022"
},
{
"article-title": "Impact of extended-course oral nirmatrelvir/ritonavir (Paxlovid) in established Long COVID: case series and research considerations",
"author": "AK Cohen",
"journal-title": "Res Sq",
"key": "pmed.1004711.ref011",
"year": "2023"
},
{
"DOI": "10.1080/14656566.2023.2262387",
"article-title": "Paxlovid as a potential treatment for Long COVID",
"author": "MW McCarthy",
"doi-asserted-by": "crossref",
"first-page": "1839",
"issue": "17",
"journal-title": "Expert Opin Pharmacother",
"key": "pmed.1004711.ref012",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1001/jamainternmed.2024.2007",
"article-title": "Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial",
"author": "LN Geng",
"doi-asserted-by": "crossref",
"first-page": "1024",
"issue": "9",
"journal-title": "JAMA Intern Med",
"key": "pmed.1004711.ref013",
"volume": "184",
"year": "2024"
},
{
"DOI": "10.1038/s41467-021-26513-3",
"article-title": "Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status",
"author": "Y Xie",
"doi-asserted-by": "crossref",
"first-page": "6571",
"issue": "1",
"journal-title": "Nat Commun",
"key": "pmed.1004711.ref014",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/ana.26649",
"article-title": "Neurologic manifestations of Long COVID differ based on acute COVID-19 severity",
"author": "GS Perez Giraldo",
"doi-asserted-by": "crossref",
"first-page": "146",
"issue": "1",
"journal-title": "Ann Neurol",
"key": "pmed.1004711.ref015",
"volume": "94",
"year": "2023"
},
{
"DOI": "10.1186/s12889-023-16916-w",
"article-title": "Risk factors associated with post-acute sequelae of SARS-CoV-2: an N3C and NIH RECOVER study",
"author": "EL Hill",
"doi-asserted-by": "crossref",
"first-page": "2103",
"issue": "1",
"journal-title": "BMC Public Health",
"key": "pmed.1004711.ref016",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.ejim.2021.06.009",
"article-title": "Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis",
"author": "C Fernández-de-Las-Peñas",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Eur J Intern Med",
"key": "pmed.1004711.ref017",
"volume": "92",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"article-title": "Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition",
"author": "Y Xie",
"doi-asserted-by": "crossref",
"first-page": "554",
"issue": "6",
"journal-title": "JAMA Intern Med",
"key": "pmed.1004711.ref018",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.7326/M23-1394",
"article-title": "Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. veterans : a target trial emulation",
"author": "GN Ioannou",
"doi-asserted-by": "crossref",
"first-page": "1486",
"issue": "11",
"journal-title": "Ann Intern Med",
"key": "pmed.1004711.ref019",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29333",
"article-title": "Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study",
"author": "MS Durstenfeld",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "J Med Virol",
"key": "pmed.1004711.ref020",
"volume": "96",
"year": "2024"
},
{
"DOI": "10.21203/rs.3.rs-3231786/v1",
"doi-asserted-by": "crossref",
"key": "pmed.1004711.ref021",
"unstructured": "Congdon S, Narrowe Z, Yone N, Gunn J, Deng Y, Nori P, et al. Nirmatrelvir/ritonavir and risk of Long COVID symptoms: a retrospective cohort study. 2023 [cited 29 Nov 2023]. https://doi.org/10.21203/rs.3.rs-3231786/v1"
},
{
"DOI": "10.1136/bmjopen-2022-069176",
"article-title": "Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community (PANORAMIC): protocol for a randomised, controlled, open-label, adaptive platform trial of community novel antiviral treatment of COVID-19 in people at increased risk of more severe disease",
"author": "O Gbinigie",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "BMJ Open",
"key": "pmed.1004711.ref022",
"volume": "13",
"year": "2023"
},
{
"key": "pmed.1004711.ref023",
"unstructured": "Canadian adaptive platform trial of treatments for COVID-19 in community settings (CanTreatCOVID). In: ClinicalTrials.gov [Internet]. 6 Jul 2023 [cited 26 Mar 2024]. Available from: https://clinicaltrials.gov/study/NCT05614349"
},
{
"DOI": "10.1093/jamia/ocaa196",
"article-title": "The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment",
"author": "MA Haendel",
"doi-asserted-by": "crossref",
"first-page": "427",
"issue": "3",
"journal-title": "J Am Med Inform Assoc",
"key": "pmed.1004711.ref024",
"volume": "28",
"year": "2021"
},
{
"key": "pmed.1004711.ref025",
"unstructured": "RECOVER: researching COVID to enhance recovery. In: RECOVER: Researching COVID to Enhance Recovery [Internet]. [cited 26 Mar 2023]. Available from: https://recovercovid.org/"
},
{
"DOI": "10.1093/jamia/ocab217",
"article-title": "Synergies between centralized and federated approaches to data quality: a report from the national COVID cohort collaborative",
"author": "ER Pfaff",
"doi-asserted-by": "crossref",
"first-page": "609",
"issue": "4",
"journal-title": "J Am Med Inform Assoc",
"key": "pmed.1004711.ref026",
"volume": "29",
"year": "2022"
},
{
"key": "pmed.1004711.ref027"
},
{
"DOI": "10.1093/aje/kwv254",
"article-title": "Using Big Data to emulate a target trial when a randomized trial is not available",
"author": "MA Hernán",
"doi-asserted-by": "crossref",
"first-page": "758",
"issue": "8",
"journal-title": "Am J Epidemiol",
"key": "pmed.1004711.ref028",
"volume": "183",
"year": "2016"
},
{
"DOI": "10.1001/jama.2022.21383",
"article-title": "Target trial emulation: a framework for causal inference from observational data",
"author": "MA Hernán",
"doi-asserted-by": "crossref",
"first-page": "2446",
"issue": "24",
"journal-title": "JAMA",
"key": "pmed.1004711.ref029",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1111/jpc.15811",
"article-title": "COVID-19 in children. II: pathogenesis, disease spectrum and management",
"author": "AR Howard-Jones",
"doi-asserted-by": "crossref",
"first-page": "46",
"issue": "1",
"journal-title": "J Paediatr Child Health",
"key": "pmed.1004711.ref030",
"volume": "58",
"year": "2022"
},
{
"article-title": "Pediatric nirmatrelvir/ritonavir prescribing patterns during the COVID-19 pandemic",
"author": "S Bose-Brill",
"journal-title": "medRxiv",
"key": "pmed.1004711.ref031",
"year": "2022"
},
{
"author": "CDC",
"key": "pmed.1004711.ref032",
"year": "2023"
},
{
"key": "pmed.1004711.ref033",
"unstructured": "Paxlovid Drug-Drug Interactions. In: COVID-19 treatment guidelines [Internet]. [cited 27 Nov 2023]. Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/"
},
{
"article-title": "Reengineering a machine learning phenotype to adapt to the changing COVID-19 landscape: a study from the N3C and RECOVER consortia",
"author": "M Crosskey",
"journal-title": "bioRxiv",
"key": "pmed.1004711.ref034",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.18931",
"article-title": "Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021",
"author": "Global Burden of Disease Long COVID Collaborators",
"doi-asserted-by": "crossref",
"first-page": "1604",
"issue": "16",
"journal-title": "JAMA",
"key": "pmed.1004711.ref035",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1212/WNL.0000000000207078",
"article-title": "An introduction to individual participant data meta-analysis",
"author": "AA Veroniki",
"doi-asserted-by": "crossref",
"first-page": "1102",
"issue": "23",
"journal-title": "Neurology",
"key": "pmed.1004711.ref036",
"volume": "100",
"year": "2023"
},
{
"article-title": "Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies",
"author": "British Medical Journal Publishing Group",
"first-page": "335",
"journal-title": "BMJ",
"key": "pmed.1004711.ref037",
"year": "2007"
},
{
"DOI": "10.1016/S1473-3099(23)00053-1",
"article-title": "Why we need a deeper understanding of the pathophysiology of Long COVID",
"author": "A Iwasaki",
"doi-asserted-by": "crossref",
"first-page": "393",
"issue": "4",
"journal-title": "Lancet Infect Dis",
"key": "pmed.1004711.ref038",
"volume": "23",
"year": "2023"
},
{
"author": "N Anschuetz",
"key": "pmed.1004711.ref039",
"year": "2023"
},
{
"DOI": "10.1161/STROKEAHA.123.042360",
"article-title": "Traumatic brain injury and long-term risk of stroke among US military veterans",
"author": "ALC Schneider",
"doi-asserted-by": "crossref",
"first-page": "2059",
"issue": "8",
"journal-title": "Stroke",
"key": "pmed.1004711.ref040",
"volume": "54",
"year": "2023"
},
{
"DOI": "10.3390/ijerph192316305",
"article-title": "Asbestos exposure and severity of COVID-19",
"author": "G Granados",
"doi-asserted-by": "crossref",
"first-page": "16305",
"issue": "23",
"journal-title": "Int J Environ Res Public Health",
"key": "pmed.1004711.ref041",
"volume": "19",
"year": "2022"
},
{
"key": "pmed.1004711.ref042",
"unstructured": "PTSD: National Center for PTSD. [cited 15 Dec 2023]. Available from: https://www.ptsd.va.gov/professional/treat/essentials/epidemiology.asp"
},
{
"author": "A Munz",
"journal-title": "Mesothelioma Center—Vital Services for Cancer Patients & Families",
"key": "pmed.1004711.ref043",
"year": "2022"
},
{
"DOI": "10.1111/j.1475-6773.2005.00448.x",
"article-title": "Measurement in Veterans Affairs Health Services Research: veterans as a special population",
"author": "RO Morgan",
"doi-asserted-by": "crossref",
"first-page": "1573",
"journal-title": "Health Serv Res",
"key": "pmed.1004711.ref044",
"volume": "40",
"year": "2005"
},
{
"DOI": "10.1016/j.jclinepi.2011.01.001",
"article-title": "Administrative database research infrequently used validated diagnostic or procedural codes",
"author": "C van Walraven",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10",
"journal-title": "J Clin Epidemiol",
"key": "pmed.1004711.ref045",
"volume": "64",
"year": "2011"
},
{
"DOI": "10.1214/09-STS313",
"article-title": "Matching methods for causal inference: a review and a look forward",
"author": "EA Stuart",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Stat Sci",
"key": "pmed.1004711.ref046",
"volume": "25",
"year": "2010"
},
{
"DOI": "10.1111/j.1467-985X.2007.00527.x",
"article-title": "Misunderstandings between experimentalists and observationalists about causal inference",
"author": "K Imai",
"doi-asserted-by": "crossref",
"first-page": "481",
"issue": "2",
"journal-title": "J R Stat Soc Ser A Stat Soc",
"key": "pmed.1004711.ref047",
"volume": "171",
"year": "2008"
},
{
"DOI": "10.1001/jamainternmed.2020.6252",
"article-title": "Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19",
"author": "S Gupta",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "JAMA Intern Med",
"key": "pmed.1004711.ref048",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.0452",
"article-title": "Estimates of overall survival in patients with cancer receiving different treatment regimens: emulating hypothetical target trials in the surveillance, epidemiology, and end results (SEER)-Medicare linked database",
"author": "LC Petito",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "pmed.1004711.ref049",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1038/s41591-019-0597-x",
"article-title": "Avoidable flaws in observational analyses: an application to statins and cancer",
"author": "BA Dickerman",
"doi-asserted-by": "crossref",
"first-page": "1601",
"issue": "10",
"journal-title": "Nat Med",
"key": "pmed.1004711.ref050",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1513/AnnalsATS.201903-241OC",
"article-title": "Emulating a novel clinical trial using existing observational data. predicting results of the PreVent study",
"author": "AJ Admon",
"doi-asserted-by": "crossref",
"first-page": "998",
"issue": "8",
"journal-title": "Ann Am Thorac Soc",
"key": "pmed.1004711.ref051",
"volume": "16",
"year": "2019"
},
{
"article-title": "A clinical case definition of post-COVID-19 condition by a Delphi consensus",
"author": "WHO Clinical Case Definition Working Group on Post-COVID-19 Condition",
"issue": "4",
"journal-title": "Lancet Infect Dis",
"key": "pmed.1004711.ref052",
"volume": "22",
"year": "2022"
},
{
"author": "Department of Health and Human Services, Office of the Assistant Secretary for Health",
"key": "pmed.1004711.ref053",
"volume-title": "National research action plan on Long COVID",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00135-7",
"article-title": "Long COVID: aiming for a consensus",
"author": "D Munblit",
"doi-asserted-by": "crossref",
"first-page": "632",
"issue": "7",
"journal-title": "Lancet Respir Med",
"key": "pmed.1004711.ref054",
"volume": "10",
"year": "2022"
},
{
"author": "EnSpark Consulting",
"key": "pmed.1004711.ref055",
"volume-title": "What we heard: engagement report on the working definition for Long COVID",
"year": "2023"
},
{
"article-title": "Strategies for handling missing data in electronic health record derived data",
"author": "BJ Wells",
"first-page": "1035",
"issue": "3",
"journal-title": "EGEMS (Wash DC)",
"key": "pmed.1004711.ref056",
"volume": "1",
"year": "2013"
},
{
"DOI": "10.1097/EDE.0000000000000794",
"article-title": "Out-of-system care and recording of patient characteristics critical for comparative effectiveness research",
"author": "KJ Lin",
"doi-asserted-by": "crossref",
"first-page": "356",
"issue": "3",
"journal-title": "Epidemiology",
"key": "pmed.1004711.ref057",
"volume": "29",
"year": "2018"
},
{
"article-title": "Evaluating risk-prediction models using data from electronic health records",
"author": "LE Wang",
"first-page": "286",
"issue": "1",
"journal-title": "Ann Appl Stat",
"key": "pmed.1004711.ref058",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1007/s42399-020-00363-4",
"article-title": "Comorbidity and its impact on patients with COVID-19",
"author": "A Sanyaolu",
"doi-asserted-by": "crossref",
"first-page": "1069",
"issue": "8",
"journal-title": "SN Compr Clin Med",
"key": "pmed.1004711.ref059",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.18632/aging.103579",
"article-title": "Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis",
"author": "X Fang",
"doi-asserted-by": "crossref",
"first-page": "12493",
"issue": "13",
"journal-title": "Aging (Albany NY)",
"key": "pmed.1004711.ref060",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1159/000513288",
"article-title": "The association between presence of comorbidities and COVID-19 severity: a systematic review and meta-analysis",
"author": "M Honardoost",
"doi-asserted-by": "crossref",
"first-page": "132",
"issue": "2",
"journal-title": "Cerebrovasc Dis",
"key": "pmed.1004711.ref061",
"volume": "50",
"year": "2021"
},
{
"key": "pmed.1004711.ref062",
"unstructured": "Palantir Foundry. In: Palantir [Internet]. [cited 9 Jan 2023]. Available from: https://www.palantir.com/platforms/foundry/"
},
{
"key": "pmed.1004711.ref063",
"unstructured": "SPH and Sharecare release community well-being rankings. [cited 30 Mar 2023]. Available from: https://www.bu.edu/sph/news/articles/2020/sph-and-sharecare-release-community-well-being-rankings/"
}
],
"reference-count": 63,
"references-count": 63,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pmed.1004711"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of Paxlovid treatment during acute COVID-19 on Long COVID onset: An EHR-based target trial emulation from the N3C and RECOVER consortia",
"type": "journal-article",
"update-policy": "https://doi.org/10.1371/journal.pmed.corrections_policy",
"update-to": [
{
"DOI": "10.1371/journal.pmed.1004711",
"label": "New version",
"source": "publisher",
"type": "new_version",
"updated": {
"date-parts": [
[
2025,
9,
18
]
],
"date-time": "2025-09-18T00:00:00Z",
"timestamp": 1758153600000
}
}
],
"updated-by": [
{
"DOI": "10.1371/journal.pmed.1004711",
"label": "New version",
"source": "publisher",
"type": "new_version",
"updated": {
"date-parts": [
[
2025,
9,
18
]
],
"date-time": "2025-09-18T00:00:00Z",
"timestamp": 1758153600000
}
}
],
"volume": "22"
}