Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo controlled randomized trial in healthcare workers
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.07.006, EPICOS, NCT04334928, Aug 2022
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
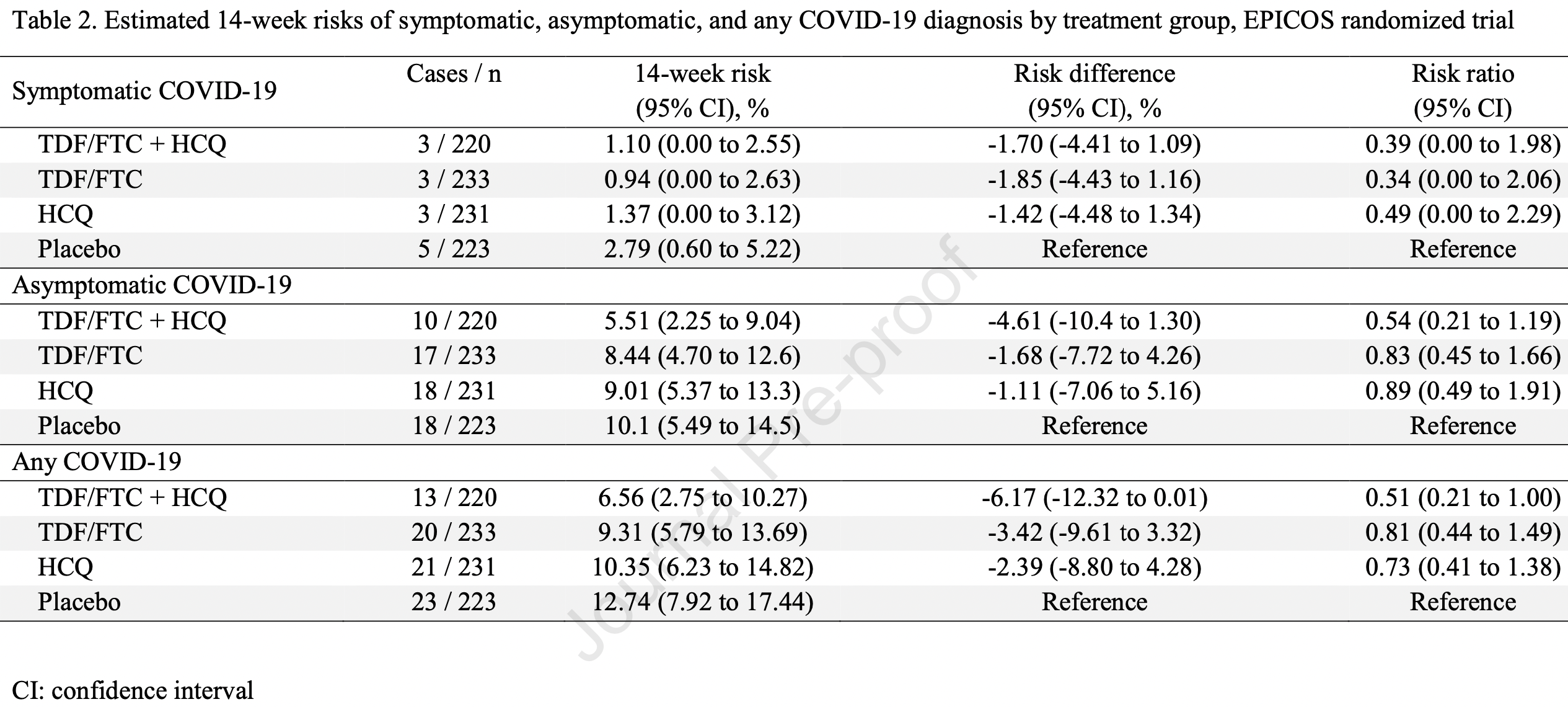
Early terminated healthcare worker prophylaxis RCT in Spain, showing lower risk of symptomatic cases with both HCQ and tenofovir disoproxil fumarate/emtricitabine, without statistical significance due to the small number of events.
|
risk of symptomatic case, 51.0% lower, RR 0.49, p = 0.79, treatment 3 of 224 (1.3%), control 5 of 223 (2.2%), Kaplan-Meier, primary outcome.
|
|
risk of case, 27.0% lower, RR 0.73, p = 0.31, treatment 21 of 231 (9.1%), control 23 of 223 (10.3%), Kaplan-Meier.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Polo et al., 5 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, median age 38.0, 27 authors, study period 15 April, 2020 - 11 July, 2021, trial NCT04334928 (history) (EPICOS).
Contact: jamo@sanidad.gob.es.
Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo-controlled randomized trial in healthcare workers
Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.07.006
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Abella, Jolkovsky, Biney, Uspal, Hyman et al., Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2
Arvelo, None
Causalab, Chan, School of Public Health
Chien, Anderson, Jockusch, Tao, Li et al., Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID
Clososki, Soldi, Da Silva, Guaratini, Lopes et al., None
Copertino, Lima, Duarte, Powell, Ormsby, None
Davis, Ferreira, Denholm, Tong, Clinical trials for the prevention and treatment of COVID-19: current state of play, Med J Aust
Del Amo, Polo, Moreno, Díaz, Martínez et al., Antiretrovirals and Risk of COVID-19 Diagnosis and Hospitalization in HIV-Positive Persons, Epidemiology
Del Amo, Polo, Moreno, Díaz, Martínez et al., Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study, Ann Intern Med
Del Amo, Polo, Moreno, Martínez, Cabello et al., None
Elfiky, Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study, Life Sci
Embi, Levy, Naleway, Patel, Gaglani et al., Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults -Nine States, January, MMWR Morb Mortal Wkly Rep
Feng, Bilello, Babusis, Gordon, Tchesnokov et al., NRTIs tenofovir, TAF, TDF, and FTC are inactive against SARS-CoV-2
García-Albéniz, Amo, Polo, Morales-Asencio, Hernán et al., Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial (HERO-HCQ), European Journal of Epidemiology
General De Segovia, -p r o o f References
Hernández-Díaz, Bateman, Straub, Zhu, Mogun et al., Safety of Tenofovir Disoproxil Fumarate for Pregnant Women Facing the Coronavirus Disease 2019 Pandemic, Am J Epidemiol
Jockusch, Tao, Li, Anderson, Chien et al., A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19, Antiviral Res
Lopez, Chiner-Oms, De Viedma, The first wave of the COVID-19 epidemic in Spain was associated with early introductions and fast spread of a dominating genetic variant, Nat Genet
Mehra, Desai, Ruschitzka, Patel, RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis, Lancet
Muñoz-Mateos, Buti, Fernández, Hernández, Bernal, Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients, Journal of Hepatology
Parienti, Prazuck, Peyro-Saint-Paul, Fournier, Valentin et al., Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal J o u r n a l P r e -p r o o f SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial, EClinicalMedicine
Park, Yu, Kim, Kim, Kim et al., Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets, mBio
Patterson, Prince, Kraft, Jenkins, Shaheen et al., Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission, Sci Transl Med
Pilkington, Hill, Hughes, Nwokolo, Pozniak, How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP, J Virus Erad
Ponticelli, Moroni, Hydroxychloroquine in systemic lupus erythematosus (SLE), Expert Opin Drug Saf
Predhomme, Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State, AIDS Res Hum Retroviruses
Rajasingham, Bangdiwala, Nicol, Skipper, Pastick et al., Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial, Clin Infect Dis
Seet, Quek, Ooi, Sengupta, Lakshminarasappa et al., Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial, Int J Infect Dis
Seifert, Chen, Meditz, Castillo-Mancilla, Gardner, None
Skipper, Pastick, Engen, Bangdiwala, Abassi et al., Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial, Ann Intern Med
Twigg, Schnizlein-Bick, Weiden, Valentine, Wheat et al., Measurement of antiretroviral drugs in the lungs of HIV-infected patients, HIV Ther
Wilkin, Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection, J Biomol Struct Dyn
Yao, Ye, Zhang, Cui, Huang et al., Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis
Zanella, Zizioli, Castelli, Quiros-Roldan, Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection
DOI record:
{
"DOI": "10.1016/j.cmi.2022.07.006",
"ISSN": [
"1198-743X"
],
"URL": "http://dx.doi.org/10.1016/j.cmi.2022.07.006",
"alternative-id": [
"S1198743X22003706"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo controlled randomized trial in healthcare workers"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Clinical Microbiology and Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.cmi.2022.07.006"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Polo",
"given": "R.",
"sequence": "first"
},
{
"affiliation": [],
"family": "García-Albéniz",
"given": "X.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terán",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morales",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rial-Crestelo",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcinuño",
"given": "M.A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García del Toro",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hita",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Sirvent",
"given": "J.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buzón",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz de Santiago",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez Arellano",
"given": "J.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanz",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bachiller",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez Alfaro",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz-Brito",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masiá",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández-Torres",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guerra",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arazo",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arribas",
"given": "J.R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez de Salazar",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernán",
"given": "M.A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del Amo",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "del Amo",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosa Polo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Santiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berenguer",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez",
"given": "Esteban",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernán",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez de Salazar",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García de Albéniz",
"given": "Xabier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iradier",
"given": "Marieta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jarrín",
"given": "Inma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamora",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivero",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Menéndez",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conde",
"given": "Enrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montes",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Terán",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores",
"given": "Bettsy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elena Choque",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peñaranda",
"given": "Jhaquelin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gorena",
"given": "Gladys",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herrera",
"given": "Mariluz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farfán",
"given": "Marcela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moya",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camacho",
"given": "Jhonny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ordoñez",
"given": "Jovanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayora",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farfán",
"given": "Brayan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morales",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benítez",
"given": "Maryelis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bolaños",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colina",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pulido",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rubio",
"given": "Rafael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bisbal",
"given": "Otilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lagarde",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Epalza",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lillo-Díaz",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez",
"given": "Raúl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sebastián Pedrodomingo",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de la Hoz",
"given": "César",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez",
"given": "Demetrio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cristina Antolí",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grande",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "María Astudillo",
"given": "Dulce",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deltoro",
"given": "Miguel García",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chirivella",
"given": "Jose Ignacio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hita",
"given": "César",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montero",
"given": "María Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruíz",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alonso",
"given": "Ma Mar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alemán",
"given": "Ma Remedios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López",
"given": "Ana Ma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García",
"given": "Dácil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelazas",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Recio",
"given": "Pablo González",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez-Vera",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De la Fuente",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vázquez",
"given": "José Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carranza",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaén",
"given": "Nieves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lavilla",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pisos",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suárez",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Ignacio de los",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Fraile",
"given": "Lucio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gutiérrez",
"given": "Ángela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bautista",
"given": "Azucena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de los Santos",
"given": "José María Alonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferreira",
"given": "Eva María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrero",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mateos",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanch",
"given": "José Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solis García",
"given": "Julian Eloy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanmartí",
"given": "Montserrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez",
"given": "Raquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Encarna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Álvarez",
"given": "María Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Abellán",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gutiérrez",
"given": "Félix",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Padilla",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estañ",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moral",
"given": "Encarnación",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marín",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roura",
"given": "Aychel Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pareja",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martín Regidor",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gutiérrez",
"given": "Esperanza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valdivia",
"given": "Luis Jorge",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Capón",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Domínguez",
"given": "Antonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Antonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruiz",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garrido",
"given": "Rubén",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cecilio",
"given": "Álvaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fenoll",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peregrina",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anguita",
"given": "Francisco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martín",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dronda",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Häemmerle",
"given": "Johannes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crespillo",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castellano",
"given": "Lidia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dueñas",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández",
"given": "Laura Rodríguez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zapico",
"given": "Genoveva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tasias",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berrocal",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Campo",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baño",
"given": "Jesús Rodríguez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Domínguez-Castellano",
"given": "Ángel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ríos-Villegas",
"given": "María José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noguera-Julian",
"given": "Antoni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fortuny",
"given": "Clàudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ríos-Barnés",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández-Pérez",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Ledesma",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carbonell",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Navarro",
"given": "Jacinto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "del Hoyo",
"given": "Cristina Sánchez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morán",
"given": "Yolanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabello",
"given": "Alfonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrillo",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Górgolas",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Antela",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Losada",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Domínguez",
"given": "Maria Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mariño",
"given": "Ana Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Trigo",
"given": "Sabela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez-Varela",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguilar",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González-Lama",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plata",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rubio",
"given": "Ma Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marín",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zardoya",
"given": "Lucía",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendoza",
"given": "Diego de",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gutiérrez",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coll",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanjoaquín",
"given": "Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loscos",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geijo",
"given": "M.P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belinchon",
"given": "O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bernal",
"given": "Enrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deschamps-Perdomo",
"given": "Ámbar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arranz",
"given": "José Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borobia",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knobel",
"given": "Hernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Artero",
"given": "Arturo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivero",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galindo",
"given": "María José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cepeda",
"given": "Concepción",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanco",
"given": "José Ramón",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estrada",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lecuona",
"given": "Ainhoa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asensi",
"given": "Víctor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cervero",
"given": "Miguel",
"sequence": "additional"
}
],
"container-title": "Clinical Microbiology and Infection",
"container-title-short": "Clinical Microbiology and Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalmicrobiologyandinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
5
]
],
"date-time": "2022-08-05T06:45:48Z",
"timestamp": 1659681948000
},
"deposited": {
"date-parts": [
[
2022,
8,
5
]
],
"date-time": "2022-08-05T11:18:03Z",
"timestamp": 1659698283000
},
"funder": [
{
"DOI": "10.13039/501100004587",
"award": [
"COV20/01112"
],
"doi-asserted-by": "publisher",
"name": "Instituto de Salud Carlos III"
},
{
"DOI": "10.13039/100016145",
"doi-asserted-by": "publisher",
"name": "Ministerio de Sanidad, Consumo y Bienestar Social"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
5
]
],
"date-time": "2022-08-05T11:41:25Z",
"timestamp": 1659699685109
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X22003706?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X22003706?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
8
]
]
},
"published-print": {
"date-parts": [
[
2022,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.5694/mja2.50673",
"article-title": "Clinical trials for the prevention and treatment of COVID-19: current state of play",
"author": "Davis",
"doi-asserted-by": "crossref",
"first-page": "86",
"issue": "2",
"journal-title": "Med J Aust",
"key": "10.1016/j.cmi.2022.07.006_bib1",
"volume": "213",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.6319",
"article-title": "Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial",
"author": "Abella",
"doi-asserted-by": "crossref",
"first-page": "195",
"issue": "2",
"journal-title": "JAMA Internal Medicine",
"key": "10.1016/j.cmi.2022.07.006_bib2",
"volume": "181",
"year": "2021"
},
{
"key": "10.1016/j.cmi.2022.07.006_bib3",
"unstructured": "García-Albéniz X, Del Amo J, Polo R, Morales-Asencio JM, Hernán MA. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. European Journal of Epidemiology, In Press."
},
{
"article-title": "Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial (HERO-HCQ)",
"author": "Naggie",
"journal-title": "medRxiv",
"key": "10.1016/j.cmi.2022.07.006_bib4",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1571",
"article-title": "Hydroxychloroquine as Pre-exposure Prophylaxis for Coronavirus Disease 2019 (COVID-19) in Healthcare Workers: A Randomized Trial",
"author": "Rajasingham",
"doi-asserted-by": "crossref",
"first-page": "e835",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.cmi.2022.07.006_bib5",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.7326/M20-3689",
"article-title": "Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study",
"author": "Del Amo",
"doi-asserted-by": "crossref",
"first-page": "536",
"issue": "7",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.cmi.2022.07.006_bib6",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1097/EDE.0000000000001235",
"article-title": "Antiretrovirals and Risk of COVID-19 Diagnosis and Hospitalization in HIV-Positive Persons",
"author": "Del Amo",
"doi-asserted-by": "crossref",
"first-page": "e49",
"issue": "6",
"journal-title": "Epidemiology",
"key": "10.1016/j.cmi.2022.07.006_bib7",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1021/acs.jproteome.0c00392",
"article-title": "Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19",
"author": "Chien",
"doi-asserted-by": "crossref",
"first-page": "4690",
"issue": "11",
"journal-title": "J Proteome Res",
"key": "10.1016/j.cmi.2022.07.006_bib8",
"volume": "19",
"year": "2020"
},
{
"article-title": "Tenofovir Disoproxil Fumarate: New Chemical Developments and Encouraging in vitro Biological Results for SARS-CoV-2",
"author": "Clososki",
"first-page": "1552",
"issue": "8",
"journal-title": "Journal of the Brazilian Chemical Society [online]",
"key": "10.1016/j.cmi.2022.07.006_bib9",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2021.1901144",
"article-title": "Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection",
"author": "Copertino",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J Biomol Struct Dyn",
"key": "10.1016/j.cmi.2022.07.006_bib10",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2020.117592",
"article-title": "Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study",
"author": "Elfiky",
"doi-asserted-by": "crossref",
"first-page": "117592",
"journal-title": "Life Sci",
"key": "10.1016/j.cmi.2022.07.006_bib11",
"volume": "253",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104857",
"article-title": "A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19",
"author": "Jockusch",
"doi-asserted-by": "crossref",
"first-page": "104857",
"journal-title": "Antiviral Res",
"key": "10.1016/j.cmi.2022.07.006_bib12",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.3390/ph14050454",
"article-title": "Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection",
"author": "Zanella",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Pharmaceuticals (Basel)",
"key": "10.1016/j.cmi.2022.07.006_bib13",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.3003174",
"article-title": "Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission",
"author": "Patterson",
"doi-asserted-by": "crossref",
"first-page": "112re4",
"issue": "112",
"journal-title": "Sci Transl Med",
"key": "10.1016/j.cmi.2022.07.006_bib14",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1089/aid.2016.0008",
"article-title": "Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State",
"author": "Seifert",
"doi-asserted-by": "crossref",
"first-page": "981",
"issue": "10-11",
"journal-title": "AIDS Res Hum Retroviruses",
"key": "10.1016/j.cmi.2022.07.006_bib15",
"volume": "32",
"year": "2016"
},
{
"DOI": "10.2217/hiv.10.5",
"article-title": "Measurement of antiretroviral drugs in the lungs of HIV-infected patients",
"author": "Twigg",
"doi-asserted-by": "crossref",
"first-page": "247",
"issue": "2",
"journal-title": "HIV Ther",
"key": "10.1016/j.cmi.2022.07.006_bib16",
"volume": "4",
"year": "2010"
},
{
"DOI": "10.1080/14740338.2017.1269168",
"article-title": "Hydroxychloroquine in systemic lupus erythematosus (SLE)",
"author": "Ponticelli",
"doi-asserted-by": "crossref",
"first-page": "411",
"issue": "3",
"journal-title": "Expert Opin Drug Saf",
"key": "10.1016/j.cmi.2022.07.006_bib17",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1016/S2055-6640(20)30312-5",
"article-title": "How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP",
"author": "Pilkington",
"doi-asserted-by": "crossref",
"first-page": "215",
"issue": "4",
"journal-title": "J Virus Erad",
"key": "10.1016/j.cmi.2022.07.006_bib18",
"volume": "4",
"year": "2018"
},
{
"DOI": "10.1093/aje/kwab109",
"article-title": "Safety of Tenofovir Disoproxil Fumarate for Pregnant Women Facing the Coronavirus Disease 2019 Pandemic",
"author": "Hernández-Díaz",
"doi-asserted-by": "crossref",
"first-page": "2339",
"issue": "11",
"journal-title": "Am J Epidemiol",
"key": "10.1016/j.cmi.2022.07.006_bib19",
"volume": "190",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"issue": "15",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.cmi.2022.07.006_bib20",
"volume": "71",
"year": "2020"
},
{
"article-title": "RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis",
"author": "Mehra",
"journal-title": "Lancet",
"key": "10.1016/j.cmi.2022.07.006_bib21",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2021.04.035",
"article-title": "Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial",
"author": "Seet",
"doi-asserted-by": "crossref",
"first-page": "314",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.cmi.2022.07.006_bib22",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1198",
"article-title": "Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa",
"doi-asserted-by": "crossref",
"first-page": "e2005",
"issue": "7",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.cmi.2022.07.006_bib23",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1101/2021.11.11.21266189",
"doi-asserted-by": "crossref",
"key": "10.1016/j.cmi.2022.07.006_bib24",
"unstructured": "Del Amo J, Polo R, Moreno S, Martínez E, Cabello A, Iribarren J, et al. Tenofovir Disoproxil Fumarate and severity of COVID-19 in people with HIV infection. 29th Conference on Retroviruses and Opportunistic Infections (CROI), February 2022; Abstract 00867."
},
{
"article-title": "Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients",
"author": "Muñoz-Mateos",
"first-page": "S746",
"journal-title": "Journal of Hepatology",
"key": "10.1016/j.cmi.2022.07.006_bib25",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1128/mBio.01114-20",
"article-title": "Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "e01114",
"issue": "3",
"journal-title": "mBio",
"key": "10.1016/j.cmi.2022.07.006_bib26",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100993",
"article-title": "Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial",
"author": "Parienti",
"doi-asserted-by": "crossref",
"first-page": "100993",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.cmi.2022.07.006_bib27",
"volume": "38",
"year": "2021"
},
{
"key": "10.1016/j.cmi.2022.07.006_bib28",
"unstructured": "Feng J, Bilello J, Babusis D, Gordon C, Tchesnokov E, Perry J, et al. NRTIs tenofovir, TAF, TDF, and FTC are inactive against SARS-CoV-2. European AIDS Conference. 2021."
},
{
"DOI": "10.15585/mmwr.mm7044e3",
"article-title": "Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults - Nine States, January-September 2021",
"author": "Embi",
"doi-asserted-by": "crossref",
"first-page": "1553",
"issue": "44",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.cmi.2022.07.006_bib29",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1038/s41588-021-00936-6",
"article-title": "The first wave of the COVID-19 epidemic in Spain was associated with early introductions and fast spread of a dominating genetic variant",
"author": "Lopez",
"doi-asserted-by": "crossref",
"first-page": "1405",
"journal-title": "Nat Genet",
"key": "10.1016/j.cmi.2022.07.006_bib30",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.7326/M20-4207",
"article-title": "Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial",
"author": "Skipper",
"doi-asserted-by": "crossref",
"first-page": "623",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.cmi.2022.07.006_bib31",
"volume": "173",
"year": "2020"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1198743X22003706"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo controlled randomized trial in healthcare workers",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
