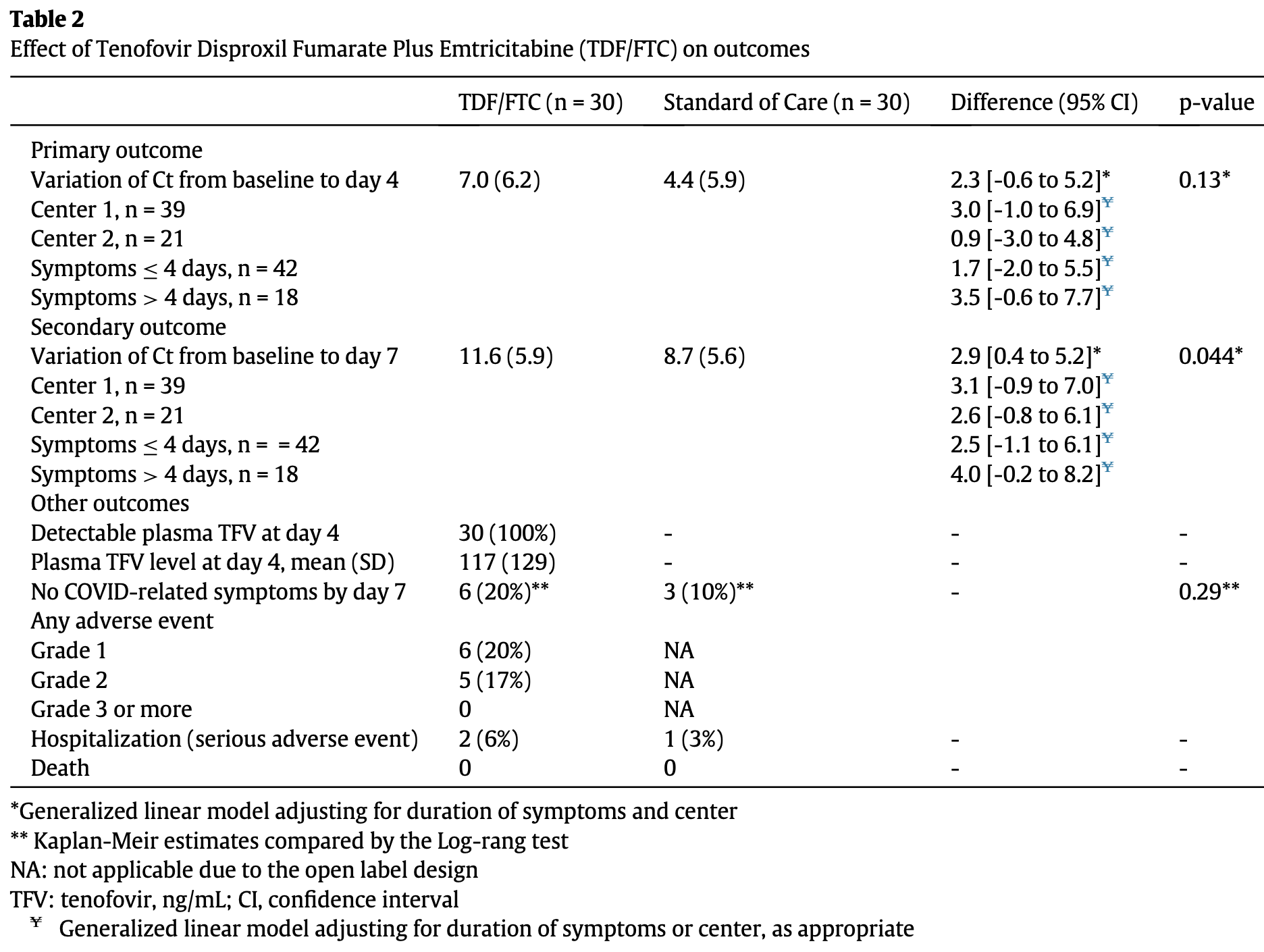
Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.100993, AR0-CORONA, NCT04685512, Aug 2021
RCT 60 outpatients with mild/moderate COVID-19 showing no significant clinical benefit with tenofovir disoproxil fumarate plus emtricitabine. The primary endpoint of viral load reduction at day 4 was not met (p=0.13), though a secondary endpoint showed viral load reduction at day 7 (p=0.044). There was no significant difference in symptom recovery time between groups.
|
risk of hospitalization, 100% higher, RR 2.00, p = 1.00, treatment 2 of 30 (6.7%), control 1 of 30 (3.3%).
|
|
risk of no recovery, 11.1% lower, RR 0.89, p = 0.47, treatment 24 of 30 (80.0%), control 27 of 30 (90.0%), NNT 10, day 7.
|
|
risk of no viral clearance, 37.1% lower, RR 0.63, p = 0.10, treatment mean 7.0 (±6.2) n=30, control mean 4.4 (±5.9) n=30, relative Ct reduction, day 4.
|
|
risk of no viral clearance, 25.0% lower, RR 0.75, p = 0.06, treatment mean 11.6 (±5.9) n=30, control mean 8.7 (±5.6) n=30, relative Ct reduction, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Parienti et al., 31 Aug 2021, Double Blind Randomized Controlled Trial, France, peer-reviewed, median age 42.0, 15 authors, study period 20 November, 2020 - 19 March, 2021, this trial uses multiple treatments in the treatment arm (combined with emtricitabine) - results of individual treatments may vary, trial NCT04685512 (history) (AR0-CORONA).
Contact: parienti-jj@chu-caen.fr.
Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial
eClinicalMedicine, doi:10.1016/j.eclinm.2021.100993
Background: Tenofovir and emtricitabine interfere with the SARS CoV-2 ribonucleic acid (RNA)-dependent RNA polymerase (RdRp). Several cohorts reported that people treated by tenofovir disoproxil fumarate and emtricitabine are less likely to develop SARS CoV-2 infection and related severe COVID-19. Methods: We conducted a pilot randomized, open-label, controlled, phase 2 trial at two hospitals in France. Eligible patients were consecutive outpatients (aged 18 years) with RT-PCR-confirmed SARS-CoV-2 infection and an interval from symptom onset to enrolment of 7 days or less. Patients were randomly assigned in a 1:1 ratio to receive oral tenofovir disoproxil fumarate and emtricitabine (2 pills on day 1 followed by 1 pill per day on days 2À7) or the standard of care. The primary and secondary endpoints were SARS-CoV-2 viral clearance from baseline assessed by cycle threshold (Ct) RT-PCR on nasopharyngeal swab collected at day 4 and day 7, respectively. A higher Ct corresponds to a lower SARS CoV-2 viral burden. Other endpoints were the time to recovery and the number of adverse events. This trial is registered with ClinicalTrials.gov, NCT04685512. Findings: From November, 20 th 2020 to March, 19 th 2021, 60 patients were enrolled and randomly assigned to a treatment group (30 to tenofovir disoproxil fumarate and emtricitabine and 30 to standard of care). The median number of days from symptom onset to inclusion was 4 days (IQR 3À5) in both groups. Amongst patients who received tenofovir disoproxil fumarate, the difference from standard of care in the increase in Ct RT-PCR from baseline was 2.3 (95% confidence interval [-0.6 to 5.2], p = 0.13) at day 4 and 2.9 (95% CI [0.1 to 5.2], p = 0.044) at day 7. At day 7, 6/30 in the tenofovir disoproxil fumarate and emtricitabine group and 3/ 30 in the standard of care group reported no COVID-related symptoms. Adverse events included 11 cases of gastrointestinal side effects (grade 2), three of which leaded to drug discontinuation. Three patients had COVID-19 related hospitalisation, no participant died. Interpretation: In this pilot study of outpatients adult with recent non-severe COVID-19, tenofovir disoproxil fumarate plus emtricitabine appeared to accelerate the natural clearance of nasopharyngeal SARS-CoV-2 viral burden. These findings support the conduct of larger trials of tenofovir-based therapies for the prevention and early treatment of COVID-19.
Supplementary materials Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100993.
References
Amo, Polo, Moreno, Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons, Epidemiology
Amo, Polo, Moreno, Incidence and Severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy : a cohort study, Ann Intern Med
Arons, Hatfield, Reddy, Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility, New England Journal of Medicine, doi:10.1056/NEJMoa2008457
Ayerdi, Puerta, Clavo, Preventive Efficacy of Tenofovir/Emtricitabine against severe acute respiratory syndrome coronavirus 2 Among Pre-Exposure Prophylaxis Users, Open Forum Infect Dis
Berenguer, Diez, Martin-Vincente, Prevalence and factors associated with SARS-CoV-2 antibodies in a SPANISH HIV Cohort
Bernal, Andrews, Gower, Effectiveness of BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on mortality following COVID-19, medR
Boulle, Davies, Hussey, Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa, Clin Infect Dis, doi:10.1093/cid/ciaa1198
Castillo-Mancilla, Meditz, Wilson, Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals, J Acquir Immune Defic Syndr
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med
Chien, Anderson, Jockusch, Nucleotide analogues as inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19, J Proteome Res
Clososki, Soldi, Silva, Da, Tenofovir Disoproxil Fumarate: New chemical developments and encouraging in vitro biological results for SARS-CoV-2, J Braz Chem Soc
Cornejo-Giraldo, Rosado, Salinas, Tenofovir-DF versus Hydroxychloroquine in the treatment of hospitalized patients with COVID-19: an observational study (TEDHICOV), medRxiv
Das, Chu, Santos, Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco, PLoS ONE
Dejong, Spinelli, Okochi, Gandhi, Tenofovir-based PrEP for COVID-19: an untapped opportunity?AIDS, doi:10.1097/QAD.0000000000002877
Dietz, The estimation of the basic reproduction number for infectious diseases, Stat Methods Med Res
Elfiky, Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study, Life Sci
Gandhi, Lynch, Rio, Mild or moderate Covid-19, N Engl J Med
Gandhi, Rutherford, Facial masking for COVID-19 -Potential for "Variolation" as we await a vaccine, New England Journal of Medicine, doi:10.1056/NEJMp2026913
Gudipati, Brar, Murray, Mckinnon, Yared et al., Descriptive analysis of patients living with HIV affected by COVID-19, J Acquir Immune Defic Syndr
Hernandez-Diaz, Bateman, Straub, Safety of Tenofovir Disoproxil Fumarate (TDF) for pregnant women facing the COVID-19 pandemic, Am J Epidemiol, doi:10.1093/aje/kwab109
Hoffmann, Arora, Groß, SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies, Cell, doi:10.1016/j.cell.2021.03.036
H€ Arter, Spinner, Roider, COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients, Infection
Isernia, Gac, SARS-COV2 infection in 30 HIV-infected patients followed-up in a French University Hospital, Int J Infect Dis
Lee, He, Eisenberg, Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue, Antimicrob Agents Chemother
Lingas, Hingrat, Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2017962118
Lorizate, Role of lipids in virus replication, Cold Spring Harb Perspect Biol
Mathew, Giles, Baxter, Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications, Science, doi:10.1126/science.abc8511
Melchjorsen, Risør, Søgaard, Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells, J Acquir Immune Defic Syndr
Mitj A, Corbacho-Monn E, Ubals, Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial, Clin Infect Dis, doi:10.1093/cid/ciaa1009
Molina, Capitant, Spire, On-demand preexposure prophylaxis in men at high risk for HIV-1 infection, N Engl J Med
Painter, Sheahan, Baric, Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with MOLNUPIRAVIR
Parienti, Bangsberg, Verdon, Gardner, Better adherence with oncedaily antiretroviral regimens: a meta-analysis, Clin Infect Dis
Park, Yu, Kim, Antiviral Efficacies of FDA-approved drugs against SARS-CoV-2 infection in Ferrets, mBio, doi:10.1128/mBio.01114-20
Phan, Nguyen, Luong, Importation and human-to-human transmission of a Novel Coronavirus in Vietnam, N Engl J Med
Skipper, Pastick, Engen, Hydroxychloroquine in non-hospitalized adults with early covid-19 : a randomized trial, Ann Intern Med
Starr, Greaney, Hilton, Deep Mutational Scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 Binding, Cell
Varga, Flammer, Steiger, Endothelial cell infection and endotheliitis in COVID-19, Lancet
Wong, Li, Spreading of COVID-19: Density matters, PLoS One
Zandi, Amblard, Musall, Repurposing nucleoside analogs for human coronaviruses, Antimicrob Agents Chemother, doi:10.1128/AAC.01652-20
Zekarias, Watson, Vidlin, Grundmark, Sex differences in reported adverse drug reactions to COVID-19 drugs in a global database of individual case safety reports, Drug Saf
Zídek, Frankov A D, Hol Y A, Activation by 9-(R)-[2-(phosphonomethoxy)propyl] adenine of chemokine (RANTES, macrophage inflammatory protein 1alpha) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1beta) production, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1016/j.eclinm.2021.100993",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2021.100993",
"alternative-id": [
"S258953702100273X"
],
"article-number": "100993",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2021.100993"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-4774-5590",
"affiliation": [],
"authenticated-orcid": false,
"family": "Parienti",
"given": "Jean-Jacques",
"sequence": "first"
},
{
"affiliation": [],
"family": "Prazuck",
"given": "Thierry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peyro-Saint-Paul",
"given": "Laure",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fournier",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valentin",
"given": "Cécile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brucato",
"given": "Sylvie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verdon",
"given": "Renaud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sève",
"given": "Aymeric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colin",
"given": "Mathilda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lesne",
"given": "Fabien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guinard",
"given": "Jérome",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3672-9974",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ar Gouilh",
"given": "Meriadeg",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1584-2457",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dina",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vabret",
"given": "Astrid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hocqueloux",
"given": "Laurent",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
6,
27
]
],
"date-time": "2021-06-27T04:20:15Z",
"timestamp": 1624767615000
},
"deposited": {
"date-parts": [
[
2022,
9,
3
]
],
"date-time": "2022-09-03T07:04:01Z",
"timestamp": 1662188641000
},
"indexed": {
"date-parts": [
[
2025,
6,
15
]
],
"date-time": "2025-06-15T12:03:10Z",
"timestamp": 1749988990961,
"version": "3.40.5"
},
"is-referenced-by-count": 49,
"issued": {
"date-parts": [
[
2021,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
1
]
],
"date-time": "2021-08-01T00:00:00Z",
"timestamp": 1627776000000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
10
]
],
"date-time": "2021-06-10T00:00:00Z",
"timestamp": 1623283200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S258953702100273X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S258953702100273X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100993",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
8
]
]
},
"published-print": {
"date-parts": [
[
2021,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.eclinm.2021.100993_bib0001",
"unstructured": "COVID-19 Map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html (accessed March 29, 2021)."
},
{
"DOI": "10.1371/journal.pone.0242398",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0002",
"unstructured": "Wong DWS, Li Y. Spreading of COVID-19: Density matters. PLoS One2020; 15: e0242398."
},
{
"DOI": "10.1056/NEJMc2001272",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0003",
"unstructured": "Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a Novel Coronavirus in Vietnam. N Engl J Med2020; 382: 872–4."
},
{
"DOI": "10.1056/NEJMoa2008457",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0004",
"unstructured": "Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England Journal of Medicine2020; published online April 24. DOI:10.1056/NEJMoa2008457."
},
{
"DOI": "10.1016/j.cell.2020.08.012",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0005",
"unstructured": "Starr TN, Greaney AJ, Hilton SK, et al. Deep Mutational Scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 Binding. Cell2020; 182: 1295-1310.e20."
},
{
"DOI": "10.1056/NEJMp2026913",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0006",
"unstructured": "Gandhi M, Rutherford GW. Facial masking for COVID-19 — Potential for “Variolation” as we await a vaccine. New England Journal of Medicine2020; published online Sept 8. DOI:10.1056/NEJMp2026913."
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0007",
"unstructured": "Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med2021; 384: 229–37."
},
{
"DOI": "10.1016/j.cell.2021.03.036",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0008",
"unstructured": "Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell2021; published online March 20. DOI:10.1016/j.cell.2021.03.036."
},
{
"DOI": "10.1371/journal.pone.0011068",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0009",
"unstructured": "Das M, Chu PL, Santos G-M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE2010; 5: e11068."
},
{
"DOI": "10.1177/096228029300200103",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0010",
"unstructured": "Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res1993; 2: 23–41."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0011",
"unstructured": "Painter W-P, Sheahan T, Baric R, et al. Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with MOLNUPIRAVIR. CROI Conference. https://www.croiconference.org/abstract/reduction-in-infectious-sars-cov-2-in-treatment-study-of-covid-19-with-molnupiravir/ (accessed May 18, 2021)."
},
{
"DOI": "10.1086/596482",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0012",
"unstructured": "Parienti J-J, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis2009; 48: 484–8."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0013",
"unstructured": "WHO model list of essential medicines. https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU2019.06 (accessed March 29, 2021)."
},
{
"DOI": "10.1016/j.lfs.2020.117592",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0014",
"unstructured": "Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci2020; 253: 117592."
},
{
"DOI": "10.1021/acs.jproteome.0c00392",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0015",
"unstructured": "Chien M, Anderson TK, Jockusch S, et al. Nucleotide analogues as inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J Proteome Res2020; 19: 4690–7."
},
{
"DOI": "10.1128/AAC.01652-20",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0016",
"unstructured": "Zandi K, Amblard F, Musall K, et al. Repurposing nucleoside analogs for human coronaviruses. Antimicrob Agents Chemother2020; 65. DOI:10.1128/AAC.01652-20."
},
{
"DOI": "10.21577/0103-5053.20200106",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0017",
"unstructured": "Clososki GC, Soldi RA, Silva RM da, et al. Tenofovir Disoproxil Fumarate: New chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc2020; 31: 1552–6."
},
{
"DOI": "10.1128/mBio.01114-20",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0018",
"unstructured": "Park S-J, Yu K-M, Kim Y-I, et al. Antiviral Efficacies of FDA-approved drugs against SARS-CoV-2 infection in Ferrets. mBio2020; 11. DOI:10.1128/mBio.01114-20."
},
{
"DOI": "10.7326/M20-3689",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0019",
"unstructured": "Del Amo J, Polo R, Moreno S, et al. Incidence and Severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy : a cohort study. Ann Intern Med2020; 173: 536–41."
},
{
"DOI": "10.1101/2020.07.02.20145185",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0020",
"unstructured": "Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis2020; published online Aug 29. DOI:10.1093/cid/ciaa1198."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0021",
"unstructured": "Berenguer J, Diez C, Martin-Vincente M, et al. Prevalence and factors associated with SARS-CoV-2 antibodies in a SPANISH HIV Cohort. CROI Conference. https://www.croiconference.org/abstract/prevalence-and-factors-associated-with-sars-cov-2-antibodies-in-a-spanish-hiv-cohort/ (accessed March 30, 2021)."
},
{
"DOI": "10.1007/s15010-020-01438-z",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0022",
"unstructured": "Härter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection2020; 48: 681–6."
},
{
"DOI": "10.1097/QAI.0000000000002450",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0023",
"unstructured": "Gudipati S, Brar I, Murray S, McKinnon JE, Yared N, Markowitz N. Descriptive analysis of patients living with HIV affected by COVID-19. J Acquir Immune Defic Syndr2020; 85: 123–6."
},
{
"DOI": "10.1016/j.ijid.2020.09.1436",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0024",
"unstructured": "Isernia V, Julia Z, Le Gac S, et al. SARS-COV2 infection in 30 HIV-infected patients followed-up in a French University Hospital. Int J Infect Dis2020; 101: 49–51."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0025",
"unstructured": "Molina J-M, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med2015; 373: 2237–46."
},
{
"DOI": "10.1093/cid/ciaa1009",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0026",
"unstructured": "Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis2020; published online July 16. DOI:10.1093/cid/ciaa1009."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0027",
"unstructured": "Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in non-hospitalized adults with early covid-19 : a randomized trial. Ann Intern Med2020; 173: 623–31."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0028",
"unstructured": "WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. a minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis2020; 20: e192–7."
},
{
"DOI": "10.1056/NEJMcp2009249",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0029",
"unstructured": "Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med2020; 383: 1757–66."
},
{
"DOI": "10.1073/pnas.2017962118",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0030",
"unstructured": "Néant N, Lingas G, Le Hingrat Q, et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci U S A2021; 118. DOI:10.1073/pnas.2017962118."
},
{
"DOI": "10.1097/EDE.0000000000001235",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0031",
"unstructured": "Del Amo J, Polo R, Moreno S, et al. Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology2020; 31: e49–51."
},
{
"DOI": "10.1093/ofid/ofaa455",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0032",
"unstructured": "Ayerdi O, Puerta T, Clavo P, et al. Preventive Efficacy of Tenofovir/Emtricitabine against severe acute respiratory syndrome coronavirus 2 Among Pre-Exposure Prophylaxis Users. Open Forum Infect Dis2020; 7: ofaa455."
},
{
"key": "10.1016/j.eclinm.2021.100993_bib0033",
"unstructured": "Bernal JL, Andrews N, Gower C, et al. Effectiveness of BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on mortality following COVID-19. medRxiv2021; 2021.05.14.21257218."
},
{
"DOI": "10.1128/AAC.49.5.1898-1906.2005",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0034",
"unstructured": "Lee WA, He G-X, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother2005; 49: 1898–906."
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0035",
"unstructured": "Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet2020; 395: 1417–8."
},
{
"DOI": "10.1101/cshperspect.a004820",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0036",
"unstructured": "Lorizate M, Kräusslich H-G. Role of lipids in virus replication. Cold Spring Harb Perspect Biol2011; 3: a004820."
},
{
"DOI": "10.1097/QAI.0b013e3182185276",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0037",
"unstructured": "Melchjorsen J, Risør MW, Søgaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr2011; 57: 265–75."
},
{
"DOI": "10.1128/AAC.45.12.3381-3386.2001",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0038",
"unstructured": "Zídek Z, Franková D, Holý A. Activation by 9-(R)-[2-(phosphonomethoxy)propyl]adenine of chemokine (RANTES, macrophage inflammatory protein 1alpha) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1beta) production. Antimicrob Agents Chemother2001; 45: 3381–6."
},
{
"DOI": "10.1097/QAI.0000000000000529",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0039",
"unstructured": "Castillo-Mancilla JR, Meditz A, Wilson C, et al. Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals. J Acquir Immune Defic Syndr2015; 68: 495–501."
},
{
"DOI": "10.1126/science.abc8511",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0040",
"unstructured": "Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science2020; 369. DOI:10.1126/science.abc8511."
},
{
"DOI": "10.1007/s40264-020-01000-8",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0041",
"unstructured": "Zekarias A, Watson S, Vidlin SH, Grundmark B. Sex differences in reported adverse drug reactions to COVID-19 drugs in a global database of individual case safety reports. Drug Saf2020; 43: 1309–14."
},
{
"DOI": "10.1101/2021.03.24.21252635",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0042",
"unstructured": "Cornejo-Giraldo M, Rosado N, Salinas J, et al. Tenofovir-DF versus Hydroxychloroquine in the treatment of hospitalized patients with COVID-19: an observational study (TEDHICOV). medRxiv2021; 2021.03.24.21252635."
},
{
"DOI": "10.1097/QAD.0000000000002877",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0043",
"unstructured": "DeJong C, Spinelli MA, Okochi H, Gandhi M. Tenofovir-based PrEP for COVID-19: an untapped opportunity?AIDS2021; published online March 11. DOI:10.1097/QAD.0000000000002877."
},
{
"DOI": "10.1093/aje/kwab109",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.100993_bib0044",
"unstructured": "Hernandez-Diaz S, Bateman BT, Straub L, et al. Safety of Tenofovir Disoproxil Fumarate (TDF) for pregnant women facing the COVID-19 pandemic. Am J Epidemiol2021; published online April 13. DOI:10.1093/aje/kwab109."
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S258953702100273X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "38"
}