Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2022.7852, HITCH, NCT04397718, Apr 2022
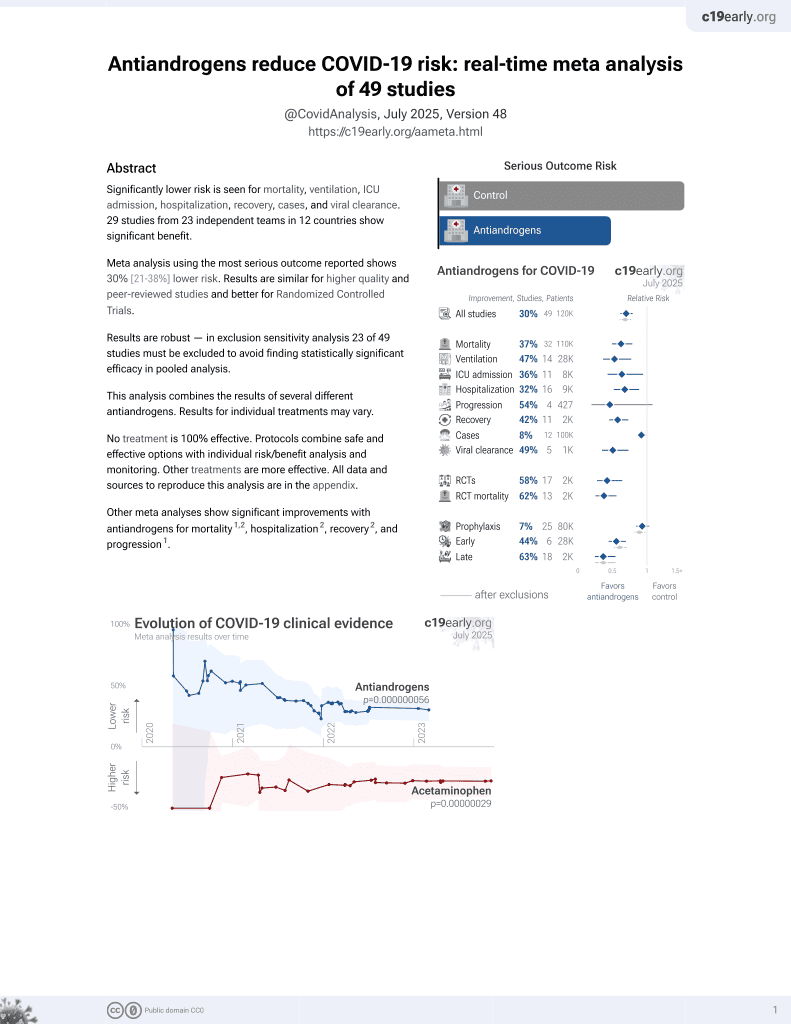
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
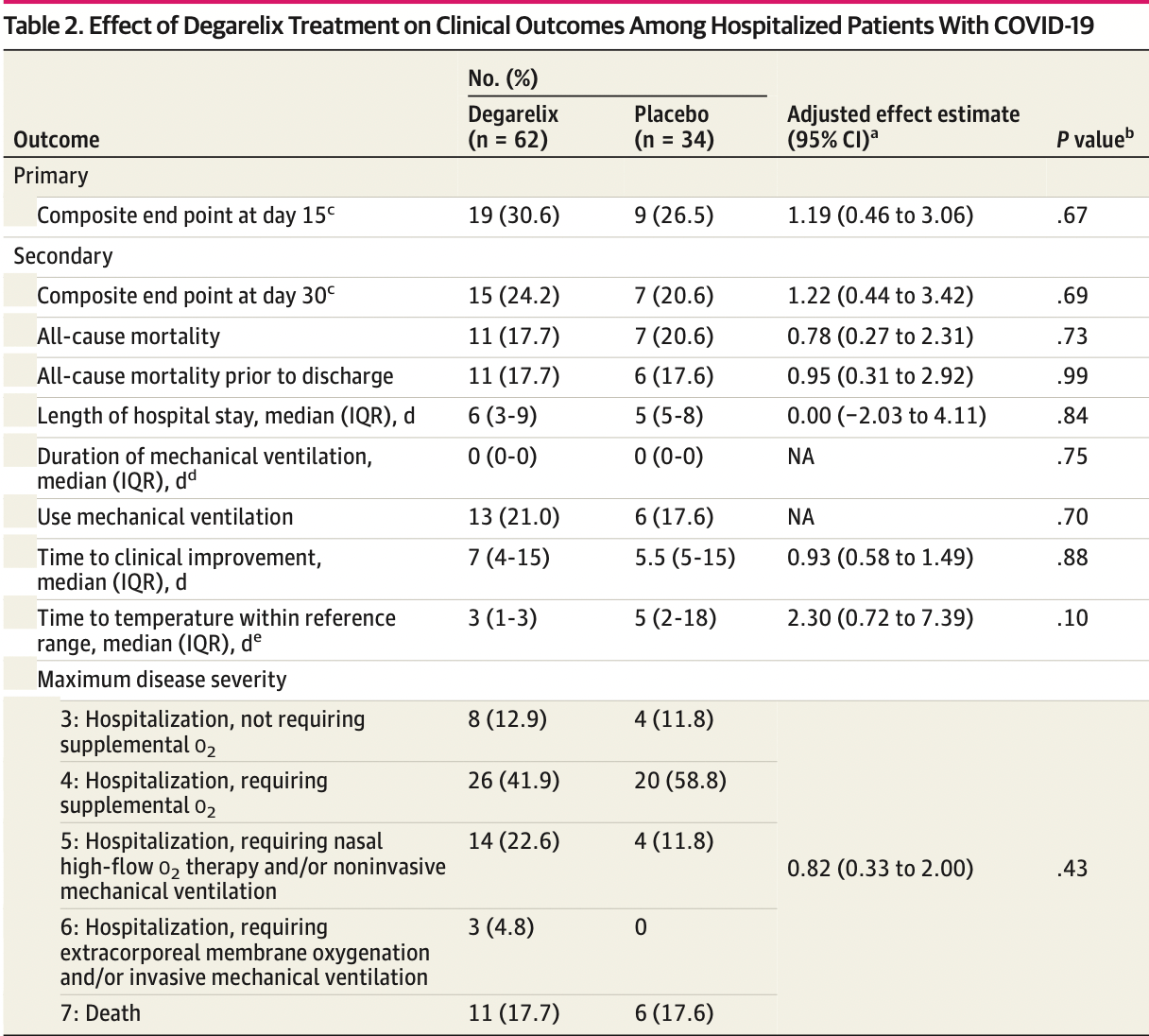
Early terminated RCT with 62 very late stage (79% on oxygen) degarelix patients and 34 placebo patients, showing no significant differences with treatment.
For discussion of many issues with this study see1.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 18.3% lower, RR 0.82, p = 0.66, treatment 11 of 62 (17.7%), control 7 of 34 (20.6%), NNT 35, adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
risk of mechanical ventilation, 18.8% higher, RR 1.19, p = 0.70, treatment 13 of 62 (21.0%), control 6 of 34 (17.6%).
|
|
risk of ongoing hospitalization, mortality, or mechanical ventilation, 16.7% higher, RR 1.17, p = 0.70, treatment 15 of 62 (24.2%), control 7 of 34 (20.6%), adjusted per study, odds ratio converted to relative risk, multivariable, primary outcome.
|
|
hospitalization time, 20.0% higher, relative time 1.20, p = 0.94, treatment 62, control 34.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Nickols et al., 19 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 34 authors, study period 22 July, 2020 - 8 April, 2021, trial NCT04397718 (history) (HITCH).
Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19
JAMA Network Open, doi:10.1001/jamanetworkopen.2022.7852
IMPORTANCE SARS-CoV-2 entry requires the TMPRSS2 cell surface protease. Antiandrogen therapies reduce expression of TMPRSS2. OBJECTIVE To determine if temporary androgen suppression induced by degarelix improves clinical outcomes of inpatients hospitalized with COVID-19. DESIGN, SETTING, AND PARTICIPANTS The Hormonal Intervention for the Treatment in Veterans With COVID-19 Requiring Hospitalization (HITCH) phase 2, placebo-controlled, double-blind, randomized clinical trial compared efficacy of degarelix plus standard care vs placebo plus standard care on clinical outcomes in men hospitalized with COVID-19 but not requiring invasive mechanical ventilation. Inpatients were enrolled at 14 Department of Veterans Affairs hospitals from July 22,
Conflict of Interest Disclosures: Dr Nickols reported receiving grants from Lantheus, Bayer, and Janssen and personal fees from Oncolinea outside the submitted work. Dr Wong reported receiving grants from the Prostate Cancer Foundation during the conduct of the study. Dr Bedimo reported receiving grants from Merck and ViiV Healthcare and personal fees from Merck, ViiV Healthcare, Janssen, Gilead Sciences, and Theratechnologies outside the submitted work. Dr Rettig reported receiving grants from Johnson & Johnson, Bayer, and Pfizer and
References
Acquisition, Nickols, Mi, Dematt, Clise et al., Critical revision of the manuscript for important intellectual content
Administrative, Nickols, Mi, Biswas, Clise et al., Supervision: Nickols
Cadegiani, Mccoy, Wambier, Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, doubleblinded, placebo-controlled trial, Cureus, doi:10.7759/cureus.13492
Caffo, Zagonel, Baldessari, On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2, Ann Oncol, doi:10.1016/j.annonc.2020.06.005
Cochran, Affairs Medical Center
Contributions, Nickols, Mi, Biswas, Clise et al., Drs Rettig and Nickols had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Davey, Grossmann, Androgen receptor structure, function and biology: from bench to bedside, Clin Biochem Rev
Deng, Rasool, Russell, Natesan, Asangani, Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19, iScience, doi:10.1016/j.isci.2021.102254
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Klein, Li, Milinovich, Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2, J Urol, doi:10.1097/JU.0000000000001338
Klotz, Boccon-Gibod, Shore, The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer, BJU Int, doi:10.1111/j.1464-410X.2008.08183.x
Mccoy, Goren, Cadegiani, Proxalutamide reduces the rate of hospitalization for covid-19 male outpatients: a randomized double-blinded placebo-controlled trial, Front Med, doi:10.3389/fmed.2021.668698
Michael, Debakey, Va, None
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532), Ann Oncol, doi:10.1016/j.annonc.2020.04.479
Nickols, Goetz, Graber, Hormonal intervention for the treatment of veterans with COVID-19 requiring hospitalization (HITCH): a multicenter, phase 2 randomized controlled trial of best supportive care vs best supportive care plus degarelix: study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-021-05389-0
O'brien, Fleming, A multiple testing procedure for clinical trials, Biometrics, doi:10.2307/2530245
Patel, Zhong, Liaw, Does androgen deprivation therapy protect against severe complications from COVID-19?, Ann Oncol, doi:10.1016/j.annonc.2020.06.023
Qiao, Wang, Mannan, Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2, Proc Natl Acad Sci, doi:10.1073/pnas.2021450118
Rick, Block, Schally, An update on the use of degarelix in the treatment of advanced hormonedependent prostate cancer, Onco Targets Ther, doi:10.2147/OTT.S32426
Schmidt, Tucker, Bakouny, Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19, JAMA Netw Open, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jamanetworkopen.2021.34330&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2022.7852
Welén, Rosendal, Gisslén, A phase 2 trial of the effect of antiandrogen therapy on COVID-19 outcome: no evidence of benefit, supported by epidemiology and in vitro data, Eur Urol, doi:10.1016/j.eururo.2021.12.013
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2022.7852",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2022.7852",
"author": [
{
"affiliation": [
{
"name": "Radiation Oncology Service, VA Greater Los Angeles Healthcare System, Los Angeles, California"
},
{
"name": "Department of Radiation Oncology, University of California, Los Angeles"
},
{
"name": "Department of Urology, University of California, Los Angeles"
}
],
"family": "Nickols",
"given": "Nicholas G.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "VA Cooperative Studies Program Coordinating Center, Perry Point, Maryland"
}
],
"family": "Mi",
"given": "Zhibao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA Cooperative Studies Program Coordinating Center, Perry Point, Maryland"
}
],
"family": "DeMatt",
"given": "Ellen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA Cooperative Studies Program Coordinating Center, Perry Point, Maryland"
}
],
"family": "Biswas",
"given": "Kousick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center, Albuquerque, New Mexico"
}
],
"family": "Clise",
"given": "Christina E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonary and Critical Care Medicine, Ralph H. Johnson VA Medical Center, Charleston, South Carolina"
},
{
"name": "Division of Pulmonary, Critical Care, Allergy and Sleep Medicine, Medical University of South Carolina, Charleston"
}
],
"family": "Huggins",
"given": "John T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medicine Service, Central Arkansas Veterans Healthcare System, Little Rock"
},
{
"name": "Division of Endocrinology and Metabolism, University of Arkansas for Medical Sciences, Little Rock"
}
],
"family": "Maraka",
"given": "Spyridoula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medicine Service, Central Arkansas Veterans Healthcare System, Little Rock"
},
{
"name": "Division of Endocrinology and Metabolism, University of Arkansas for Medical Sciences, Little Rock"
}
],
"family": "Ambrogini",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Critical Care and Sleep, College of Medicine–Jacksonville, University of Florida, Jacksonville"
}
],
"family": "Mirsaeidi",
"given": "Mehdi S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Endocrinology, Long Beach VA Medical Center, Long Beach, California"
},
{
"name": "Division of Endocrinology, Department of Medicine, University of California, Irvine"
}
],
"family": "Levin",
"given": "Ellis R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology and Oncology VA New York Harbor Healthcare System, Manhattan Campus, New York"
},
{
"name": "Perlmutter Cancer Center, NYU Langone Medical Center, New York, New York"
}
],
"family": "Becker",
"given": "Daniel J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA New York Harbor Healthcare System, Manhattan Campus, New York"
},
{
"name": "NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Makarov",
"given": "Danil V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA New York Harbor Healthcare System, Manhattan Campus, New York"
},
{
"name": "NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Adorno Febles",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA New York Harbor Healthcare System, Brooklyn Campus, Brooklyn"
}
],
"family": "Belligund",
"given": "Pooja M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA New York Harbor Healthcare System, Brooklyn Campus, Brooklyn"
}
],
"family": "Al-Ajam",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veterans Affairs Medical Center, Memphis, Tennessee"
},
{
"name": "University of Tennessee Health Science Center, Memphis"
}
],
"family": "Muthiah",
"given": "Muthiah P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology and Oncology, VA Puget Sound Health Care System, Seattle, Washington"
},
{
"name": "Division of Medical Oncology, Department of Medicine, University of Washington, Seattle"
}
],
"family": "Montgomery",
"given": "Robert B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology and Oncology, Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania"
},
{
"name": "Division of Hematology-Oncology, Perelman School of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Robinson",
"given": "Kyle W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology and Oncology, Corporal Michael J. Crescenz VA Medical Center, Philadelphia, Pennsylvania"
},
{
"name": "Division of Hematology-Oncology, Perelman School of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Wong",
"given": "Yu-Ning",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA North Texas Health Care System, Dallas"
},
{
"name": "UT Southwestern Medical Center, School of Medicine, Dallas, Texas"
}
],
"family": "Bedimo",
"given": "Roger J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Michael E. DeBakey VA Medical Center, Houston, Texas"
}
],
"family": "Villareal",
"given": "Reina C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonary and Critical Care Medicine, Phoenix VA Health Care System, Phoenix, Arizona"
}
],
"family": "Aguayo",
"given": "Samuel M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "John Cochran Veterans Affairs Medical Center, St Louis, Missouri"
},
{
"name": "Department of Medicine, Saint Louis University School of Medicine, St Louis, Missouri"
}
],
"family": "Schoen",
"given": "Martin W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Section, VA Greater Los Angeles Healthcare System, Los Angeles, California"
},
{
"name": "Department of Medicine, University of California, Los Angeles"
}
],
"family": "Goetz",
"given": "Matthew B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Section, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Graber",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Section, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Bhattacharya",
"given": "Debika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonary, Critical Care and Sleep Section, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Soo Hoo",
"given": "Guy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of California, Los Angeles"
},
{
"name": "Clinical Informatics, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Orshansky",
"given": "Greg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "VA Cooperative Studies Program Coordinating Center, Perry Point, Maryland"
}
],
"family": "Norman",
"given": "Leslie E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology-Oncology, Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Tran",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology-Oncology, Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Ghayouri",
"given": "Leila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology-Oncology, Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Tsai",
"given": "Sonny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology-Oncology, Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, California"
}
],
"family": "Geelhoed",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology-Oncology, Department of Medicine, VA Greater Los Angeles Healthcare System, Los Angeles, California"
},
{
"name": "Departments of Medicine and Urology, University of California, Los Angeles"
}
],
"family": "Rettig",
"given": "Mathew B.",
"sequence": "additional"
}
],
"container-title": [
"JAMA Network Open"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T15:13:57Z",
"timestamp": 1650381237000
},
"deposited": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T15:14:14Z",
"timestamp": 1650381254000
},
"indexed": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T15:40:51Z",
"timestamp": 1650382851531
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2574-3805"
}
],
"issue": "4",
"issued": {
"date-parts": [
[
2022,
4,
19
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2022,
4,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2791293/nickols_2022_oi_220246_1649859914.21644.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e227852",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
4,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
19
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor.",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "zoi220246r1",
"volume": "181",
"year": "2020"
},
{
"article-title": "Androgen receptor structure, function and biology: from bench to bedside.",
"author": "Davey",
"first-page": "3",
"issue": "1",
"journal-title": "Clin Biochem Rev",
"key": "zoi220246r2",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1016/j.isci.2021.102254",
"article-title": "Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19.",
"author": "Deng",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "iScience",
"key": "zoi220246r3",
"volume": "24",
"year": "2021"
},
{
"article-title": "Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2.",
"author": "Qiao",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "zoi220246r4",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532).",
"author": "Montopoli",
"doi-asserted-by": "publisher",
"first-page": "1040",
"issue": "8",
"journal-title": "Ann Oncol",
"key": "zoi220246r5",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"article-title": "Does androgen deprivation therapy protect against severe complications from COVID-19?",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "1419",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "zoi220246r6",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.005",
"article-title": "On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2.",
"author": "Caffo",
"doi-asserted-by": "publisher",
"first-page": "1415",
"issue": "10",
"journal-title": "Ann Oncol",
"key": "zoi220246r7",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1097/JU.0000000000001338",
"article-title": "Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2.",
"author": "Klein",
"doi-asserted-by": "publisher",
"first-page": "441",
"issue": "2",
"journal-title": "J Urol",
"key": "zoi220246r8",
"volume": "205",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.34330",
"article-title": "Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19.",
"author": "Schmidt",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "JAMA Netw Open",
"key": "zoi220246r9",
"volume": "4",
"year": "2021"
},
{
"article-title": "Proxalutamide reduces the rate of hospitalization for covid-19 male outpatients: a randomized double-blinded placebo-controlled trial.",
"author": "McCoy",
"journal-title": "Front Med (Lausanne)",
"key": "zoi220246r10",
"volume": "8",
"year": "2021"
},
{
"article-title": "Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial.",
"author": "Cadegiani",
"issue": "2",
"journal-title": "Cureus",
"key": "zoi220246r11",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.eururo.2021.12.013",
"article-title": "A phase 2 trial of the effect of antiandrogen therapy on COVID-19 outcome: no evidence of benefit, supported by epidemiology and in vitro data.",
"author": "Welén",
"doi-asserted-by": "publisher",
"first-page": "285",
"issue": "3",
"journal-title": "Eur Urol",
"key": "zoi220246r12",
"volume": "81",
"year": "2022"
},
{
"DOI": "10.2147/OTT.S32426",
"article-title": "An update on the use of degarelix in the treatment of advanced hormone-dependent prostate cancer.",
"author": "Rick",
"doi-asserted-by": "crossref",
"first-page": "391",
"journal-title": "Onco Targets Ther",
"key": "zoi220246r13",
"volume": "6",
"year": "2013"
},
{
"DOI": "10.1186/s13063-021-05389-0",
"article-title": "Hormonal intervention for the treatment of veterans with COVID-19 requiring hospitalization (HITCH): a multicenter, phase 2 randomized controlled trial of best supportive care vs best supportive care plus degarelix: study protocol for a randomized controlled trial.",
"author": "Nickols",
"doi-asserted-by": "publisher",
"first-page": "431",
"issue": "1",
"journal-title": "Trials",
"key": "zoi220246r14",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1111/bju.2008.102.issue-11",
"article-title": "The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer.",
"author": "Klotz",
"doi-asserted-by": "publisher",
"first-page": "1531",
"issue": "11",
"journal-title": "BJU Int",
"key": "zoi220246r15",
"volume": "102",
"year": "2008"
},
{
"DOI": "10.2307/2530245",
"article-title": "A multiple testing procedure for clinical trials.",
"author": "O’Brien",
"doi-asserted-by": "publisher",
"first-page": "549",
"issue": "3",
"journal-title": "Biometrics",
"key": "zoi220246r16",
"volume": "35",
"year": "1979"
},
{
"DOI": "10.4158/EP14003.OR",
"article-title": "Low testosterone levels are frequent in patients with acute respiratory failure and are associated with poor outcomes.",
"author": "Almoosa",
"doi-asserted-by": "publisher",
"first-page": "1057",
"issue": "10",
"journal-title": "Endocr Pract",
"key": "zoi220246r17",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1186/cc12386",
"article-title": "Etiology of low testosterone levels in male patients with severe sepsis requiring mechanical ventilation.",
"author": "Bech",
"doi-asserted-by": "publisher",
"first-page": "448",
"journal-title": "Crit Care",
"key": "zoi220246r18",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.3389/fendo.2021.694083",
"article-title": "Testosterone deficiency is a risk factor for severe COVID-19.",
"author": "Lanser",
"doi-asserted-by": "crossref",
"journal-title": "Front Endocrinol (Lausanne)",
"key": "zoi220246r19",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2018.00794",
"article-title": "Androgen-induced immunosuppression.",
"author": "Gubbels Bupp",
"doi-asserted-by": "publisher",
"first-page": "794",
"journal-title": "Front Immunol",
"key": "zoi220246r20",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.cellimm.2015.02.004",
"article-title": "Suppressive effects of androgens on the immune system.",
"author": "Trigunaite",
"doi-asserted-by": "publisher",
"first-page": "87",
"issue": "2",
"journal-title": "Cell Immunol",
"key": "zoi220246r21",
"volume": "294",
"year": "2015"
},
{
"DOI": "10.3389/fimmu.2020.01184",
"article-title": "Influence of androgens on immunity to self and foreign: effects on immunity and cancer.",
"author": "Ben-Batalla",
"doi-asserted-by": "publisher",
"first-page": "1184",
"journal-title": "Front Immunol",
"key": "zoi220246r22",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00153.2020",
"article-title": "Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells.",
"author": "Stelzig",
"doi-asserted-by": "publisher",
"first-page": "L1280",
"issue": "6",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "zoi220246r23",
"volume": "318",
"year": "2020"
},
{
"DOI": "10.14814/phy2.14707",
"article-title": "17ß-estradiol reduces SARS-CoV-2 infection in vitro.",
"author": "Lemes",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Physiol Rep",
"key": "zoi220246r24",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.eururo.2015.01.027",
"article-title": "Long-term efficacy and safety of enzalutamide monotherapy in hormone-naïve prostate cancer: 1- and 2-year open-label follow-up results.",
"author": "Tombal",
"doi-asserted-by": "publisher",
"first-page": "787",
"issue": "5",
"journal-title": "Eur Urol",
"key": "zoi220246r25",
"volume": "68",
"year": "2015"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2791293"
}
},
"score": 1,
"short-container-title": [
"JAMA Netw Open"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"The HITCH Randomized Clinical Trial"
],
"title": [
"Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19"
],
"type": "journal-article",
"volume": "5"
}