Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial
et al., Cureus, doi:10.7759/cureus.13492, Feb 2021
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 234 mild-moderate COVID-19 patients with 171 treated with proxalutamide, showing significantly faster viral clearance and recovery. Third party analysis suggests potential randomization failure:1.
This study is excluded in meta-analysis:
potential randomization failure.
|
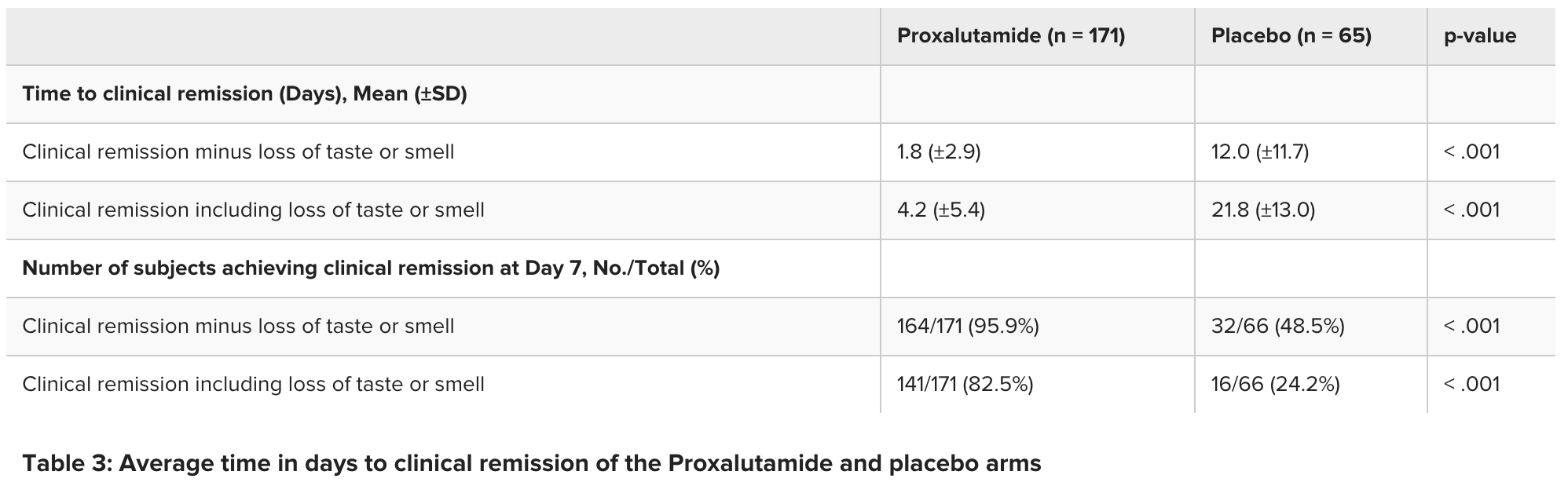
risk of no viral clearance, 92.1% lower, RR 0.08, p < 0.001, treatment 7 of 171 (4.1%), control 34 of 66 (51.5%), NNT 2.1, day 7, not including loss of taste or smell.
|
|
risk of no recovery, 76.8% lower, RR 0.23, p < 0.001, treatment 30 of 171 (17.5%), control 50 of 66 (75.8%), NNT 1.7, day 7, including loss of taste or smell.
|
|
recovery time, 85.0% lower, relative time 0.15, treatment 171, control 66, day 7, not including loss of taste or smell.
|
|
recovery time, 80.7% lower, relative time 0.19, treatment 171, control 66, day 7, including loss of taste or smell.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cadegiani et al., 22 Feb 2021, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, 8 authors, average treatment delay 4.2 days.
Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial
Cureus, doi:10.7759/cureus.13492
Background The entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into type II pneumocytes is dependent on a modification of viral spike proteins by transmembrane protease serine 2 (TMPRSS2) expressed on the surface of human cells. TMPRSS2 is regulated by the androgen receptor, hence, SARS-CoV-2 infectivity is indirectly dependent on androgenic status and phenotype. Previously, we have reported that men affected by androgenetic alopecia (AGA) are overrepresented in severe coronavirus disease 2019 . Additionally, we have reported that men taking antiandrogenic drugs, e.g., 5-alpha-reductase inhibitors (5ARis), are less likely to have severe COVID-19. Here we aimed to test whether the androgen receptor antagonist, Proxalutamide, would be a beneficial treatment for subjects with SARS-CoV-2 infection.
Methods Male and female subjects were recruited to a double-blinded, randomized, prospective, investigational study of Proxalutamide for the treatment of COVID-19. Mild to moderate, non-hospitalized subjects, who were confirmed positive for SARS-CoV-2, were treated with either Proxalutamide 200 mg/day or placebo. Endpoints for the study were remission time (days) and the percentage of subjects confirmed negative for SARS-CoV-2 on Day 7 after treatment. A negative SARS-CoV-2 test was defined by concentration-time (Ct)>40 determined by real-time reverse transcription-polymerase chain reaction (rtPCR).
Results Two-hundred thirty-six (2360 subjects were included in the study (108 female, 128 male); 171 were randomized to the Proxalutamide arm and 65 were in the placebo group. On Day 7, SARS-CoV-2 became non-detectable with rtPCR (cT>40) in 82% of the subjects in the Proxalutamide group versus 31% in the placebo group (p < 0.001). The average clinical remission time for patients treated with Proxalutamide was 4.2 ±5.4 days versus 21.8 ±13.0 days in the placebo arm (p < 0.001).
Conclusion Proxalutamide significantly accelerated viral clearance on Day 7 in mild to moderate COVID-19 patients versus placebo. Further, the time to clinical remission was significantly reduced in patients treated with Proxalutamide versus placebo.
Additional Information In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Cadegiani, Lim, Goren, Clinical symptoms of hyperandrogenic women diagnosed with COVID-19, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17004
Goren, Vaño-Galván, Wambier, A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain -a potential clue to the role of androgens in COVID-19 severity, J Cosmet Dermatol, doi:10.1111/jocd.13443
Goren, Wambier, Herrera, Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16953.10.1111/jdv.16953
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Lucas, Heinlein, Kim, The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis, Cancer Discov, doi:10.1158/2159-8290.CD-13-1010
Mallett, Allen, Graziadio, At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data, BMC Med, doi:10.1186/s12916-020-01810-8
Mccoy, Cadegiani, Wambier, 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17021
Mccoy, Wambier, Herrera, Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16956
Qu, Gu, Wang, Metabolomic profiling to evaluate the efficacy of Proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells, Invest New Drugs
Ravikirti, Pattadar, Ivermectin as a potential treatment for mild to moderate COVID-19 -a double blind randomized placebo-controlled trial, PREPRINT, doi:10.1101/2021.01.05.21249310
Rocco, Silva, Cruz, Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial, Eur Resp J Jan, doi:10.1183/13993003.03725-2020
Wambier, Vaño-Galván, Mccoy, Androgenetic alopecia present in the majority of hospitalized COVID-19 patients -the "Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.05.079
Wambier, Vaño-Galván, Mccoy, Pai, Dhurat et al., Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.07.099
Zhou, Xu, Zhang, Preclinical profile and phase I clinical trial of a novel androgen receptor antagonist GT0918 in castration-resistant prostate cancer, Eur J Cancer, doi:10.1016/j.ejca.2020.04.013
DOI record:
{
"DOI": "10.7759/cureus.13492",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.13492",
"author": [
{
"affiliation": [],
"family": "Cadegiani",
"given": "Flavio A",
"sequence": "first"
},
{
"affiliation": [],
"family": "McCoy",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gustavo Wambier",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaño-Galván",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shapiro",
"given": "Jerry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tosti",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zimerman",
"given": "Ricardo A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goren",
"given": "Andy",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
22
]
],
"date-time": "2021-02-22T17:37:11Z",
"timestamp": 1614015431000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T19:34:20Z",
"timestamp": 1707507260000
},
"indexed": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T14:39:25Z",
"timestamp": 1712155165593
},
"is-referenced-by-count": 24,
"issued": {
"date-parts": [
[
2021,
2,
22
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/52299-proxalutamide-significantly-accelerates-viral-clearance-and-reduces-time-to-clinical-remission-in-patients-with-mild-to-moderate-covid-19-results-from-a-randomized-double-blinded-placebo-controlled-trial",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2021,
2,
22
]
]
},
"published-print": {
"date-parts": [
[
2021,
2,
22
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann M",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "ref1",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, et al.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020, 181:271-280. 10.1016/j.cell.2020.02.052",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1158/2159-8290.CD-13-1010",
"article-title": "The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis",
"author": "Lucas JM",
"doi-asserted-by": "publisher",
"journal-title": "Cancer Discov",
"key": "ref2",
"unstructured": "Lucas JM, Heinlein C, Kim T, et al.. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014, 4:1310-1325. 10.1158/2159-8290.CD-13-1010",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.1016/j.jaad.2020.05.079",
"article-title": "Androgenetic alopecia present in the majority of hospitalized COVID-19 patients - the \"Gabrin sign\"",
"author": "Wambier CG",
"doi-asserted-by": "publisher",
"journal-title": "J Am Acad Dermatol",
"key": "ref3",
"unstructured": "Wambier CG, Vaño-Galván S, McCoy J, et al.. Androgenetic alopecia present in the majority of hospitalized COVID-19 patients - the \"Gabrin sign\". J Am Acad Dermatol. 2020, 83:680-682. 10.1016/j.jaad.2020.05.079",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1111/jocd.13443",
"article-title": "A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain - a potential clue to the role of androgens in COVID-19 severity",
"author": "Goren A",
"doi-asserted-by": "publisher",
"journal-title": "J Cosmet Dermatol",
"key": "ref4",
"unstructured": "Goren A, Vaño-Galván S, Wambier CG, et al.. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain - a potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020, 19:1545-1547. 10.1111/jocd.13443",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.07.099",
"article-title": "Androgenetic alopecia in COVID- 19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign",
"author": "Wambier CG",
"doi-asserted-by": "publisher",
"journal-title": "J Am Acad Dermatol",
"key": "ref5",
"unstructured": "Wambier CG, Vaño-Galván S, McCoy J, Pai S, Dhurat R, Goren A. Androgenetic alopecia in COVID- 19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. J Am Acad Dermatol. 2020, 83:E453-E454. 10.1016/j.jaad.2020.07.099",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1111/jdv.17004",
"article-title": "Clinical symptoms of hyperandrogenic women diagnosed with COVID-19",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref6",
"unstructured": "Cadegiani FA, Lim RK, Goren A, et al.. Clinical symptoms of hyperandrogenic women diagnosed with COVID-19. J Eur Acad Dermatol Venereol. 2020, 35:e101-e104. 10.1111/jdv.17004",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16953. 10.1111/jdv.16953",
"article-title": "Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men",
"author": "Goren A",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref7",
"unstructured": "Goren A, Wambier CG, Herrera S, et al.. Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men. J Eur Acad Dermatol Venereol. 2020, 25:e13-e15. 10.1111/jdv.16953. 10.1111/jdv.16953",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1111/jdv.17021",
"article-title": "5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia",
"author": "McCoy J",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref8",
"unstructured": "McCoy J, Cadegiani FA, Wambier CG, et al.. 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia. J Eur Acad Dermatol Venereol. 2020, [Epub ahead of print]:10.1111/jdv.17021",
"volume": "[Epub ahead of print]",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16956",
"article-title": "Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients",
"author": "McCoy J",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref9",
"unstructured": "McCoy J, Wambier CG, Herrera S, et al.. Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients. J Eur Acad Dermatol Venereol. 2020 Sep, 25:10.1111/jdv.16956",
"volume": "25",
"year": "2020"
},
{
"article-title": "Metabolomic profiling to evaluate the efficacy of Proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells",
"author": "Qu F",
"journal-title": "Invest New Drugs",
"key": "ref10",
"unstructured": "Qu F, Gu Y, Wang Q, et al.. Metabolomic profiling to evaluate the efficacy of Proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells. Invest New Drugs. 2020, 38:1292-1302.",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.ejca.2020.04.013",
"article-title": "Preclinical profile and phase I clinical trial of a novel androgen receptor antagonist GT0918 in castration-resistant prostate cancer",
"author": "Zhou T",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Cancer",
"key": "ref11",
"unstructured": "Zhou T, Xu W, Zhang W, et al.. Preclinical profile and phase I clinical trial of a novel androgen receptor antagonist GT0918 in castration-resistant prostate cancer. Eur J Cancer. 2020, 134:29-40. 10.1016/j.ejca.2020.04.013",
"volume": "134",
"year": "2020"
},
{
"DOI": "10.1183/13993003.03725-2020",
"article-title": "Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial",
"author": "Rocco PRM",
"doi-asserted-by": "publisher",
"journal-title": "Eur Resp J Jan",
"key": "ref12",
"unstructured": "Rocco PRM, Silva PL, Cruz FF, et al.. Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Resp J Jan. 2020, 2020:2003725. 10.1183/13993003.03725-2020",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1186/s12916-020-01810-8",
"article-title": "At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data",
"author": "Mallett S",
"doi-asserted-by": "publisher",
"journal-title": "BMC Med",
"key": "ref13",
"unstructured": "Mallett S, Allen AJ, Graziadio S, et al.. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020, 18:346. 10.1186/s12916-020-01810-8",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1101/2021.01.05.21249310",
"article-title": "Ivermectin as a potential treatment for mild to moderate COVID-19 - a double blind randomized placebo-controlled trial [PREPRINT]",
"author": "Ravikirti",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref14",
"unstructured": "Ravikirti, Roy R, Pattadar C, et al.. Ivermectin as a potential treatment for mild to moderate COVID-19 - a double blind randomized placebo-controlled trial [PREPRINT]. medRxiv. 2021, 10.1101/2021.01.05.21249310",
"year": "2021"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/52299-proxalutamide-significantly-accelerates-viral-clearance-and-reduces-time-to-clinical-remission-in-patients-with-mild-to-moderate-covid-19-results-from-a-randomized-double-blinded-placebo-controlled-trial"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial",
"type": "journal-article"
}
