Prevalence of Potential Drug Interactions With Direct-Acting Antivirals for COVID-19 Among Hospitalized Patients
et al., Clinical Therapeutics, doi:10.1016/j.clinthera.2024.08.004, Sep 2024
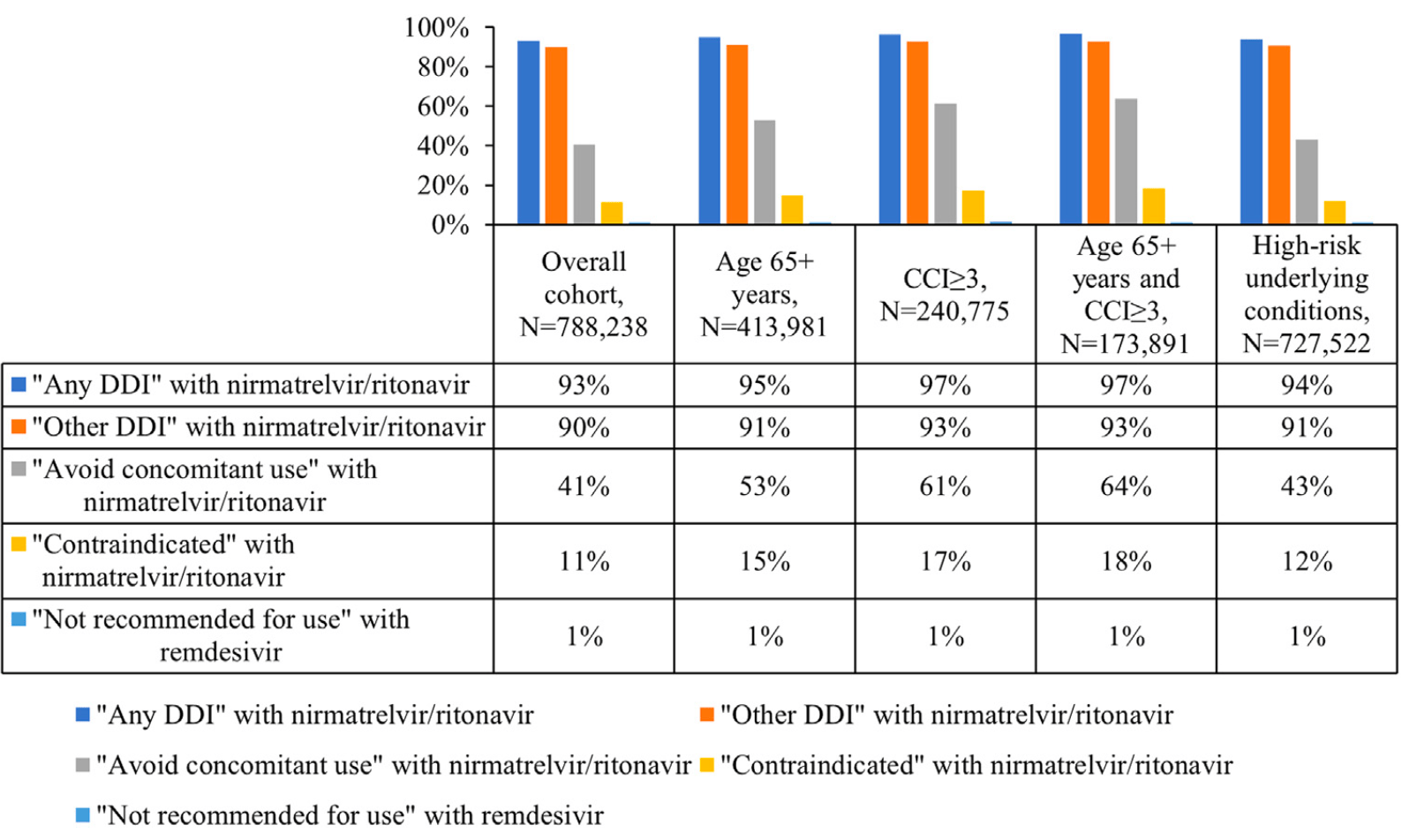
Retrospective 788,238 hospitalized COVID-19 patients in the US, showing a very high prevalence of drug-drug interactions with paxlovid, with higher prevalence for older patients, patients with more comorbidities, and patients at high-risk for severe COVID-19.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Mozaffari et al., 7 Sep 2024, retrospective, USA, peer-reviewed, 9 authors, study period May 2020 - December 2022.
Contact: rivera.christina@mayo.edu.
Prevalence of Potential Drug Interactions With Direct-Acting Antivirals for COVID-19 Among Hospitalized Patients
Clinical Therapeutics, doi:10.1016/j.clinthera.2024.08.004
Implications: A significant proportion of patients hospitalized for COVID-19 receive medications for other conditions that have the potential to result in DDIs with DAAs; most predominantly with nirmatrelvir/ritonavir, a strong CYP3A enzyme inhibitor, fewer with remdesivir, and none with molnupiravir. Higher age and comorbidity burden were significantly associated with a higher likelihood of receiving medications that are "Contraindicated " with nirmatrelvir/ritonavir. In the evolving COVID-19 era, these findings provide insights into patients hospitalized for COVID-19, and the polypharmacy evaluations that clinicians may encounter when selecting among DAAs to manage COVID-19.
Declaration of competing interest Summary of ICMJE forms: Essy Mozaffari is an employee of Gilead Sciences, Inc. and holds stock or stock options. Aastha Chandak is an employee of Certara and declares support provided by Gilead Sciences, Inc. to her institution. Andrew Ustianowski declares consulting fees provided by Generate Biomedicines, Gilead Sciences, Inc., Merck/MSD, and Pfizer Inc. and payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events, and/or participation on a Data Safety Monitoring Board or Advisory Board from Astra Zeneca, Gilead Sciences, Inc., and GSK. Christina G. Rivera declares the receipt of grant or contracts from Gilead Science, Inc. as payment to PI colleague, consulting fees and participation on a Data Safety Monitoring Board or Advisory Board from Gilead Science, Inc., and payment of honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Jobson Health, Continuing Education Network, CE Impact, Insmed, Gilead Sciences, Inc., Annenberg Center for Health Science, American College of Clinical Pharmacy, and PharmCon Healthcare Education. Neera Ahuja declares payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Gilead Sciences, Inc. Heng Jiang is an employee of Certara and declares support provided by Gilead Sciences, Inc. to his institution. Mark Berry is an employee of Gilead Sciences, Inc...
References
Abraham, Nohria, Neilan, Cardiovascular drug interactions with nirmatrelvir/ritonavir in patients with COVID-19: JACC review topic of the week, J Am Coll Cardiol
Bigdelou, Sepand, Najafikhoshnoo, COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes, Front Immunol
Carpenter, Berry, Pelletier, Clinically relevant drug-drug interactions in primary care, Am Fam Physician
Chan, Current and future direct-acting antivirals against COVID-19, Front Microbiol
Chang, Shariff, Bakar, Polypharmacy and potentially inappropriate medications among hospitalized older adults with COVID-19 in Malaysian tertiary hospitals, J Pharm Policy Pract
Day, Snowden, Mclachlan, Life-threatening drug interactions: what the physician needs to know, Intern Med J
Ghasemi, Darvishi, Salari, Global prevalence of polypharmacy among the COVID-19 patients: a comprehensive systematic review and meta-analysis of observational studies, Trop Med Health
Iloanusi, Mgbere, Essien, Polypharmacy among COVID-19 patients: a systematic review, J Am Pharm Assoc
Kumar, Trivedi, Disease-drug and drug-drug interaction in COVID-19: risk and assessment, Biomed Pharmacother
Larsen, Assessing the proportion of the Danish population at risk of clinically significant drug-drug interactions with new oral antivirals for early treatment of COVID-19, Int J Infect Dis
Lemaitre, Grégoire, Monchaud, Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for COVID-19: guidelines from the French Society of Pharmacology and Therapeutics (SFPT), Therapie
Marzolini, Kuritzkes, Marra, Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlodid) and comedications, Clin Pharmacol Ther
Russell, Lone, Baillie, Comorbidities, multimorbidity and COVID-19, ARTICLE IN PRESS JID: CLITHE [m5GeSdc
Singh, De, Antiviral agents for the treatment of COVID-19: progress and challenges, Cell Rep Med
Sirois, Boiteau, Chiu, Exploring the associations between polypharmacy and COVID-19-related hospitalisations and deaths: a population-based cohort study among older adults in Quebec, Canada, BMJ Open
Who, COVID-19: WHO health emergency appeal
DOI record:
{
"DOI": "10.1016/j.clinthera.2024.08.004",
"ISSN": [
"0149-2918"
],
"URL": "http://dx.doi.org/10.1016/j.clinthera.2024.08.004",
"alternative-id": [
"S0149291824002157"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Prevalence of Potential Drug Interactions With Direct-Acting Antivirals for COVID-19 Among Hospitalized Patients"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Clinical Therapeutics"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.clinthera.2024.08.004"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Mozaffari",
"given": "Essy",
"sequence": "first"
},
{
"affiliation": [],
"family": "Chandak",
"given": "Aastha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8308-3264",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rivera",
"given": "Christina G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahuja",
"given": "Neera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Heng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berry",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okulicz",
"given": "Jason F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amin",
"given": "Alpesh N.",
"sequence": "additional"
}
],
"container-title": "Clinical Therapeutics",
"container-title-short": "Clinical Therapeutics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicaltherapeutics.com",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
7
]
],
"date-time": "2024-09-07T10:29:38Z",
"timestamp": 1725704978000
},
"deposited": {
"date-parts": [
[
2024,
9,
7
]
],
"date-time": "2024-09-07T10:29:59Z",
"timestamp": 1725704999000
},
"funder": [
{
"DOI": "10.13039/100005564",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100005564",
"id-type": "DOI"
}
],
"name": "Gilead Sciences Inc"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
8
]
],
"date-time": "2024-09-08T00:26:02Z",
"timestamp": 1725755162978
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
20
]
],
"date-time": "2024-08-20T00:00:00Z",
"timestamp": 1724112000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0149291824002157?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0149291824002157?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
9
]
]
},
"published-print": {
"date-parts": [
[
2024,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.clinthera.2024.08.004_bib0001",
"unstructured": "CDC. COVID data tracker. 2024. https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions. Accessed March 15, 2024."
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0002",
"unstructured": "WHO. COVID-19: WHO health emergency appeal. 2024. https://www.who.int/publications/m/item/covid-19-who-health-emergency-appeal-2024. Accessed January 16, 2024."
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0003",
"unstructured": "CDC. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed February 6, 2024."
},
{
"DOI": "10.3389/fimmu.2022.890517",
"article-title": "COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes",
"author": "Bigdelou",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "10.1016/j.clinthera.2024.08.004_bib0004",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-02156-9",
"article-title": "Comorbidities, multimorbidity and COVID-19",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "334",
"journal-title": "Nat Med",
"key": "10.1016/j.clinthera.2024.08.004_bib0005",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1186/s40545-022-00504-1",
"article-title": "Polypharmacy and potentially inappropriate medications among hospitalized older adults with COVID-19 in Malaysian tertiary hospitals",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "J Pharm Policy Pract",
"key": "10.1016/j.clinthera.2024.08.004_bib0006",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1186/s41182-022-00456-x",
"article-title": "Global prevalence of polypharmacy among the COVID-19 patients: a comprehensive systematic review and meta-analysis of observational studies",
"author": "Ghasemi",
"doi-asserted-by": "crossref",
"first-page": "60",
"journal-title": "Trop Med Health",
"key": "10.1016/j.clinthera.2024.08.004_bib0007",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1016/j.japh.2021.05.006",
"article-title": "Polypharmacy among COVID-19 patients: a systematic review",
"author": "Iloanusi",
"doi-asserted-by": "crossref",
"first-page": "e14",
"journal-title": "J Am Pharm Assoc (2003)",
"key": "10.1016/j.clinthera.2024.08.004_bib0008",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2021-060295",
"article-title": "Exploring the associations between polypharmacy and COVID-19-related hospitalisations and deaths: a population-based cohort study among older adults in Quebec, Canada",
"author": "Sirois",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/j.clinthera.2024.08.004_bib0009",
"volume": "12",
"year": "2022"
},
{
"article-title": "Antiviral agents for the treatment of COVID-19: progress and challenges",
"author": "Singh",
"journal-title": "Cell Rep Med",
"key": "10.1016/j.clinthera.2024.08.004_bib0010",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.3389/fmicb.2020.587944",
"article-title": "Current and future direct-acting antivirals against COVID-19",
"author": "Chan",
"doi-asserted-by": "crossref",
"journal-title": "Front Microbiol",
"key": "10.1016/j.clinthera.2024.08.004_bib0011",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2021.111642",
"article-title": "Disease-drug and drug-drug interaction in COVID-19: risk and assessment",
"author": "Kumar",
"doi-asserted-by": "crossref",
"journal-title": "Biomed Pharmacother",
"key": "10.1016/j.clinthera.2024.08.004_bib0012",
"volume": "139",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlodid) and comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.clinthera.2024.08.004_bib0013",
"volume": "112",
"year": "2022"
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0014",
"unstructured": "FDA. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. 2024. https://www.fda.gov/media/155050/download. Accessed February 10, 2024."
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0015",
"unstructured": "FDA. Remdesivir prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf. Accessed February 10, 2024."
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0016",
"unstructured": "FDA. Fact sheet for healthcare providers: emergency use authorization for Lagevrio. 2023. https://www.fda.gov/media/155054/download. Accessed February 10, 2024."
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0017",
"unstructured": "FDA. Antimicrobial Drugs Advisory Committee Meeting (16 March 2023). 2023. https://www.fda.gov/media/166237/download. Accessed February 10, 2024."
},
{
"DOI": "10.1016/j.ijid.2022.06.059",
"article-title": "Assessing the proportion of the Danish population at risk of clinically significant drug-drug interactions with new oral antivirals for early treatment of COVID-19",
"author": "Larsen",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.clinthera.2024.08.004_bib0018",
"volume": "122",
"year": "2022"
},
{
"DOI": "10.1016/j.therap.2022.03.005",
"article-title": "Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for COVID-19: guidelines from the French Society of Pharmacology and Therapeutics (SFPT)",
"author": "Lemaitre",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "Therapie",
"key": "10.1016/j.clinthera.2024.08.004_bib0019",
"volume": "77",
"year": "2022"
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0020",
"unstructured": "FDA. Paxlovid prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217188s000lbl.pdf. Accessed March 7, 2024."
},
{
"DOI": "10.1016/j.jacc.2022.08.800",
"article-title": "Cardiovascular drug interactions with nirmatrelvir/ritonavir in patients with COVID-19: JACC review topic of the week",
"author": "Abraham",
"doi-asserted-by": "crossref",
"first-page": "1912",
"journal-title": "J Am Coll Cardiol",
"key": "10.1016/j.clinthera.2024.08.004_bib0021",
"volume": "80",
"year": "2022"
},
{
"article-title": "Clinically relevant drug-drug interactions in primary care",
"author": "Carpenter",
"first-page": "558",
"journal-title": "Am Fam Physician",
"key": "10.1016/j.clinthera.2024.08.004_bib0022",
"volume": "99",
"year": "2019"
},
{
"DOI": "10.1111/imj.13404",
"article-title": "Life-threatening drug interactions: what the physician needs to know",
"author": "Day",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Intern Med J",
"key": "10.1016/j.clinthera.2024.08.004_bib0023",
"volume": "47",
"year": "2017"
},
{
"key": "10.1016/j.clinthera.2024.08.004_bib0024",
"unstructured": "NIH. Drug-drug interactions between ritonavir-boosted nirmatrelvir (Paxlovid) and concomitant medications. 2024. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir–paxlovid-/paxlovid-drug-drug-interactions/. Accessed February 12, 2024."
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0149291824002157"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Prevalence of Potential Drug Interactions With Direct-Acting Antivirals for COVID-19 Among Hospitalized Patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}