Clinical Efficacy of the Neutralizing Antibody Therapy Sotrovimab in Patients with SARS-CoV-2 Omicron BA.1 and BA.2 Subvariant Infections
et al., Viruses, doi:10.3390/v15061300, May 2023
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 844 patients treated with sotrovimab and matched controls in Japan, showing lower risk of oxygen therapy with treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending sotrovimab also recommended them, or
because the patient seeking out sotrovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.14-6, BA.4, BA.57, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.18, and no efficacy for BA.29, XBB, XBB.1.5, ХВВ.1.9.110, XBB.1.16, BQ.1.1.45, and CL.18. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments11.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of mechanical ventilation, 60.0% lower, RR 0.40, p = 0.45, treatment 2 of 844 (0.2%), control 5 of 844 (0.6%), NNT 281, all.
|
|
risk of mechanical ventilation, 33.3% lower, RR 0.67, p = 1.00, treatment 2 of 642 (0.3%), control 3 of 642 (0.5%), NNT 642, BA.1.
|
|
risk of mechanical ventilation, 80.0% lower, RR 0.20, p = 0.50, treatment 0 of 202 (0.0%), control 2 of 202 (1.0%), NNT 101, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), BA.2.
|
|
risk of oxygen therapy, 55.3% lower, RR 0.45, p < 0.001, treatment 34 of 844 (4.0%), control 76 of 844 (9.0%), NNT 20, all.
|
|
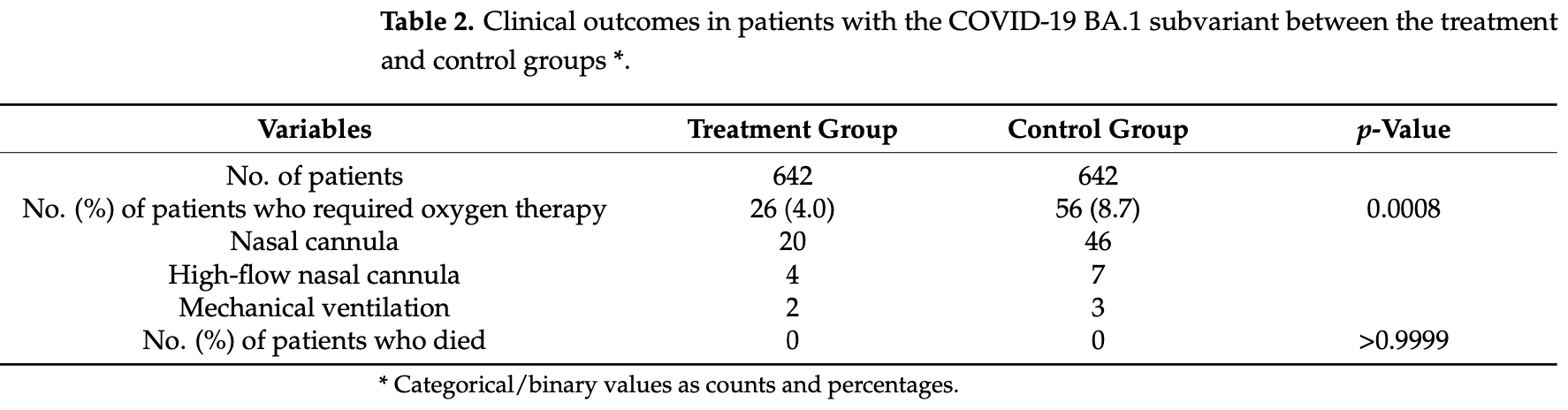
risk of oxygen therapy, 53.6% lower, RR 0.46, p < 0.001, treatment 26 of 642 (4.0%), control 56 of 642 (8.7%), NNT 21, BA.1.
|
|
risk of oxygen therapy, 60.0% lower, RR 0.40, p = 0.03, treatment 8 of 202 (4.0%), control 20 of 202 (9.9%), NNT 17, BA.2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
8.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
9.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Miyashita et al., 31 May 2023, retrospective, Japan, peer-reviewed, 7 authors, study period December 2021 - July 2022.
Contact: miyashin@hirakata.kmu.ac.jp (corresponding author), ogatam@hirakata.kmu.ac.jp, fukudana@hirakata.kmu.ac.jp, yamuraak@hirakata.kmu.ac.jp, itot@hirakata.kmu.ac.jp, 99nakamori@gmail.com, ishiuray@takii.kmu.ac.jp.
Clinical Efficacy of the Neutralizing Antibody Therapy Sotrovimab in Patients with SARS-CoV-2 Omicron BA.1 and BA.2 Subvariant Infections
Viruses, doi:10.3390/v15061300
Sotrovimab, an antibody active against severe acute respiratory syndrome coronavirus 2 that neutralizes antibodies, reduced the risk of COVID-19-related hospitalization or death in studies conducted before the emergence of the Omicron variant. The objective of this study is to evaluate the clinical efficacy of sotrovimab in patients with mild to moderate COVID-19 Omicron BA.1 and BA.2 subvariant infections using a propensity score matching method. The propensity score-matched cohort study population was derived from patients who received sotrovimab. We derived a comparator group from an age-and sex-matched population who were recuperating in a medical facility after COVID-19 infection or from elderly person entrance facilities during the same period who were eligible for but did not receive sotrovimab treatment. In total, 642 patients in the BA.1 subvariant group and 202 in the BA.2 subvariant group and matched individuals were analyzed. The outcome was the requirement for oxygen therapy. In the treatment group, 26 patients with the BA.1 subvariant and 8 patients with the BA.2 subvariant received oxygen therapy. The administration of oxygen therapy was significantly lower in the treatment group than in the control group (BA.1 subvariant group, 4.0% vs. 8.7%, p = 0.0008; BA.2 subvariant group, 4.0% vs. 9.9%, p = 0.0296). All these patients were admitted to our hospitals and received additional therapy and then recovered. No deaths were observed in either group. Our results demonstrate that the sotrovimab antibody treatment may be associated with a reduction in the requirement for oxygen therapy among high-risk patients with mild to moderate COVID-19 Omicron BA.1 and BA.2 subvariants.
Author Contributions: All the authors conceived the study, participated in its design and coordination, and collected and managed data, including quality control. N.M. and Y.N. drafted the manuscript, and all authors contributed substantially to its revision. All authors have read and agreed to the published version of the manuscript. Funding: This research received no external funding.
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee at Kansai Medical University and all participating facilities (protocol code 2020319 and 24 August 2021). Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
References
Addetia, Piccoli, Case, Park, Beltramello et al., Therapeutic and vaccine-induced cross-reactive antibodies with effector function against emerging Omicron variants, doi:10.1101/2023.01.17.523798
Aggarwal, Beaty, Bennett, Carlson, Davis et al., Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients, J. Infect. Dis, doi:10.1093/infdis/jiac206
Bruel, Stéfic, Nguyen, Toniutti, Staropoli et al., Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies, Cell Rep. Med, doi:10.1016/j.xcrm.2022.100850
Case, Mackin, Errico, Chong, Madden et al., Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains, Nat. Commun, doi:10.1038/s41467-022-31615-7
Cathcart, Havenar-Daughton, Lempp, Ma, Schmid et al., The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2, doi:10.1101/2021.03.09.434607
Cheng, Reyes, Satram, Birch, Gibbons et al., Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA, Infect. Dis. Ther, doi:10.1007/s40121-022-00755-0
Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., COMET-ICE Investigators. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N. Engl. J. Med, doi:10.1056/NEJMoa2107934
Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., COMET-ICE Investigators. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Huang, Mccreary, Bariola, Minnier, Wadas et al., Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge. A cohort study and randomized comparative effectiveness trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2022.20957
Iketani, Liu, Guo, Liu, Chan et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature, doi:10.1038/s41586-022-04594-4
Martin-Blondel, Marcelin, Soulié, Kaisaridi, Lusivika-Nzinga et al., Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2, J. Infect, doi:10.1016/j.jinf.2022.06.033
Meschi, Matusali, Colavita, Lapa, Bordi et al., Predicting the protective humoral response to a SARS-CoV-2 mRNA vaccine, Clin. Chem. Lab. Med, doi:10.1515/cclm-2021-0700
Ong, Ren, Lee, Sutjipto, Dugan et al., Real-world use of sotrovimab for pre-emptive treatment in high-risk hospitalized COVID-19 patients: An observational cross-sectional study, Antibiotics, doi:10.3390/antibiotics11030345
Park, Pinto, Walls, Liu, De Marco et al., Imprinted antibody responses against SARS-CoV-2 Omicron sublineages, doi:10.1101/2022.05.08.491108
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant, N. Engl. J. Med, doi:10.1056/NEJMc2119407
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2, N. Engl. J. Med, doi:10.1056/NEJMc2201933
Uraki, Kiso, Imai, Yamayoshi, Ito et al., Therapeutic efficacy of monoclonal antibodies and antivirals against SARS-CoV-2 Omicron BA.1 in Syrian hamsters, Nature Microbiol, doi:10.1038/s41564-022-01170-4
Zaqout, Almaslamani, Chemaitelly, Hashim, Ittaman et al., Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar, Int. J. Infect. Dis, doi:10.1016/j.ijid.2022.09.023
Zheng, Green, Tazere, Curtis, Fisher et al., Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: Observational cohort study with the OpenSAFELY platform, BMJ, doi:10.1136/bmj-2022-071932
DOI record:
{
"DOI": "10.3390/v15061300",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v15061300",
"abstract": "<jats:p>Sotrovimab, an antibody active against severe acute respiratory syndrome coronavirus 2 that neutralizes antibodies, reduced the risk of COVID-19-related hospitalization or death in studies conducted before the emergence of the Omicron variant. The objective of this study is to evaluate the clinical efficacy of sotrovimab in patients with mild to moderate COVID-19 Omicron BA.1 and BA.2 subvariant infections using a propensity score matching method. The propensity score-matched cohort study population was derived from patients who received sotrovimab. We derived a comparator group from an age- and sex-matched population who were recuperating in a medical facility after COVID-19 infection or from elderly person entrance facilities during the same period who were eligible for but did not receive sotrovimab treatment. In total, 642 patients in the BA.1 subvariant group and 202 in the BA.2 subvariant group and matched individuals were analyzed. The outcome was the requirement for oxygen therapy. In the treatment group, 26 patients with the BA.1 subvariant and 8 patients with the BA.2 subvariant received oxygen therapy. The administration of oxygen therapy was significantly lower in the treatment group than in the control group (BA.1 subvariant group, 4.0% vs. 8.7%, p = 0.0008; BA.2 subvariant group, 4.0% vs. 9.9%, p = 0.0296). All these patients were admitted to our hospitals and received additional therapy and then recovered. No deaths were observed in either group. Our results demonstrate that the sotrovimab antibody treatment may be associated with a reduction in the requirement for oxygen therapy among high-risk patients with mild to moderate COVID-19 Omicron BA.1 and BA.2 subvariants.</jats:p>",
"alternative-id": [
"v15061300"
],
"author": [
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Infectious Disease and Allergology, First Department of Internal Medicine, Kansai Medical University, 2-3-1 Shin-machi, Hirakata 573-1191, Japan"
}
],
"family": "Miyashita",
"given": "Naoyuki",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Kansai Medical University Medical Center, Moriguchi 570-8507, Japan"
}
],
"family": "Nakamori",
"given": "Yasushi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Infectious Disease and Allergology, First Department of Internal Medicine, Kansai Medical University, 2-3-1 Shin-machi, Hirakata 573-1191, Japan"
}
],
"family": "Ogata",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Infectious Disease and Allergology, First Department of Internal Medicine, Kansai Medical University, 2-3-1 Shin-machi, Hirakata 573-1191, Japan"
}
],
"family": "Fukuda",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Infectious Disease and Allergology, First Department of Internal Medicine, Kansai Medical University, 2-3-1 Shin-machi, Hirakata 573-1191, Japan"
}
],
"family": "Yamura",
"given": "Akihisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Oncology and Allergology, First Department of Internal Medicine, Kansai Medical University Medical Center, Moriguchi 570-8507, Japan"
}
],
"family": "Ishiura",
"given": "Yoshihisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Infectious Disease and Allergology, First Department of Internal Medicine, Kansai Medical University, 2-3-1 Shin-machi, Hirakata 573-1191, Japan"
}
],
"family": "Ito",
"given": "Tomoki",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T05:40:56Z",
"timestamp": 1685598056000
},
"deposited": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T05:43:51Z",
"timestamp": 1685598231000
},
"indexed": {
"date-parts": [
[
2023,
6,
2
]
],
"date-time": "2023-06-02T04:26:10Z",
"timestamp": 1685679970557
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2023,
5,
31
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2023,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
5,
31
]
],
"date-time": "2023-05-31T00:00:00Z",
"timestamp": 1685491200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/15/6/1300/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1300",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
5,
31
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "COMET-ICE Investigators. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N. Engl. J. Med.",
"key": "ref_1",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "COMET-ICE Investigators. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "ref_2",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20957",
"article-title": "Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge. A cohort study and randomized comparative effectiveness trial",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "e2220957",
"journal-title": "JAMA Netw. Open",
"key": "ref_3",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiac206",
"article-title": "Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "2129",
"journal-title": "J. Infect. Dis.",
"key": "ref_4",
"volume": "226",
"year": "2022"
},
{
"DOI": "10.3390/antibiotics11030345",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Ong, S.W.X., Ren, D., Lee, P.H., Sutjipto, S., Dugan, C., Khoo, B.Y., Tay, J.X., Vasoo, S., Young, B.E., and Lyeet, D.C. (2022). Real-world use of sotrovimab for pre-emptive treatment in high-risk hospitalized COVID-19 patients: An observational cross-sectional study. Antibiotics, 11."
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N. Engl. J. Med.",
"key": "ref_6",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41564-022-01170-4",
"article-title": "Therapeutic efficacy of monoclonal antibodies and antivirals against SARS-CoV-2 Omicron BA.1 in Syrian hamsters",
"author": "Uraki",
"doi-asserted-by": "crossref",
"first-page": "1252",
"journal-title": "Nature Microbiol.",
"key": "ref_7",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N. Engl. J. Med.",
"key": "ref_8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 Omicron sublineages",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Nature",
"key": "ref_9",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1101/2021.03.09.434607",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Cathcart, A.L., Havenar-Daughton, C., Lempp, F.A., Ma, D., Schmid, M.A., Agostini, M.L., Guarino, B., Di Iulio, J., Rosen, L.E., and Tucker, H. (2022). The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2 (version 12). bioRxiv."
},
{
"DOI": "10.1101/2022.05.08.491108",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Park, Y.J., Pinto, D., Walls, A.C., Liu, Z., De Marco, A., Benigni, F., Zatta, F., Silacci-Fregni, C., Bassi, J., and Sprouse, K.R. (2022). Imprinted antibody responses against SARS-CoV-2 Omicron sublineages (version 4). bioRxiv."
},
{
"DOI": "10.1038/s41467-022-31615-7",
"article-title": "Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains",
"author": "Case",
"doi-asserted-by": "crossref",
"first-page": "3824",
"journal-title": "Nat. Commun.",
"key": "ref_12",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.xcrm.2022.100850",
"article-title": "Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "100850",
"journal-title": "Cell Rep. Med.",
"key": "ref_13",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1515/cclm-2021-0700",
"article-title": "Predicting the protective humoral response to a SARS-CoV-2 mRNA vaccine",
"author": "Meschi",
"doi-asserted-by": "crossref",
"first-page": "2010",
"journal-title": "Clin. Chem. Lab. Med.",
"key": "ref_14",
"volume": "59",
"year": "2021"
},
{
"key": "ref_15",
"unstructured": "ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group (2022). Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): A randomised controlled trial. Lancet Infect. Dis., 22, 622–635."
},
{
"DOI": "10.1007/s40121-022-00755-0",
"article-title": "Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "607",
"journal-title": "Infect. Dis. Ther.",
"key": "ref_16",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-071932",
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: Observational cohort study with the OpenSAFELY platform",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "e071932",
"journal-title": "BMJ",
"key": "ref_17",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.06.033",
"article-title": "Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2",
"author": "Marcelin",
"doi-asserted-by": "crossref",
"first-page": "e104",
"journal-title": "J. Infect.",
"key": "ref_18",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2022.09.023",
"article-title": "Effectiveness of the neutralizing antibody sotrovimab among high-risk patients with mild-to-moderate SARS-CoV-2 in Qatar",
"author": "Zaqout",
"doi-asserted-by": "crossref",
"first-page": "96",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_19",
"volume": "124",
"year": "2022"
},
{
"DOI": "10.1101/2023.01.17.523798",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Addetia, A., Piccoli, L., Case, J.B., Park, Y.J., Beltramello, M., Guarino, B., Dang, H., Pinto, D., Scheaffer, S.M., and Sprouse, K. (2023). Therapeutic and vaccine-induced cross-reactive antibodies with effector function against emerging Omicron variants (version 2). bioRxiv."
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/15/6/1300"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "Clinical Efficacy of the Neutralizing Antibody Therapy Sotrovimab in Patients with SARS-CoV-2 Omicron BA.1 and BA.2 Subvariant Infections",
"type": "journal-article",
"volume": "15"
}
