Efficacy of single-dose and double-dose ivermectin early treatment in preventing progression to hospitalization in mild COVID-19: A multi-arm, parallel-group randomized, double-blind, placebo-controlled trial
et al., Respirology, doi:10.1111/resp.14318, Jun 2022
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
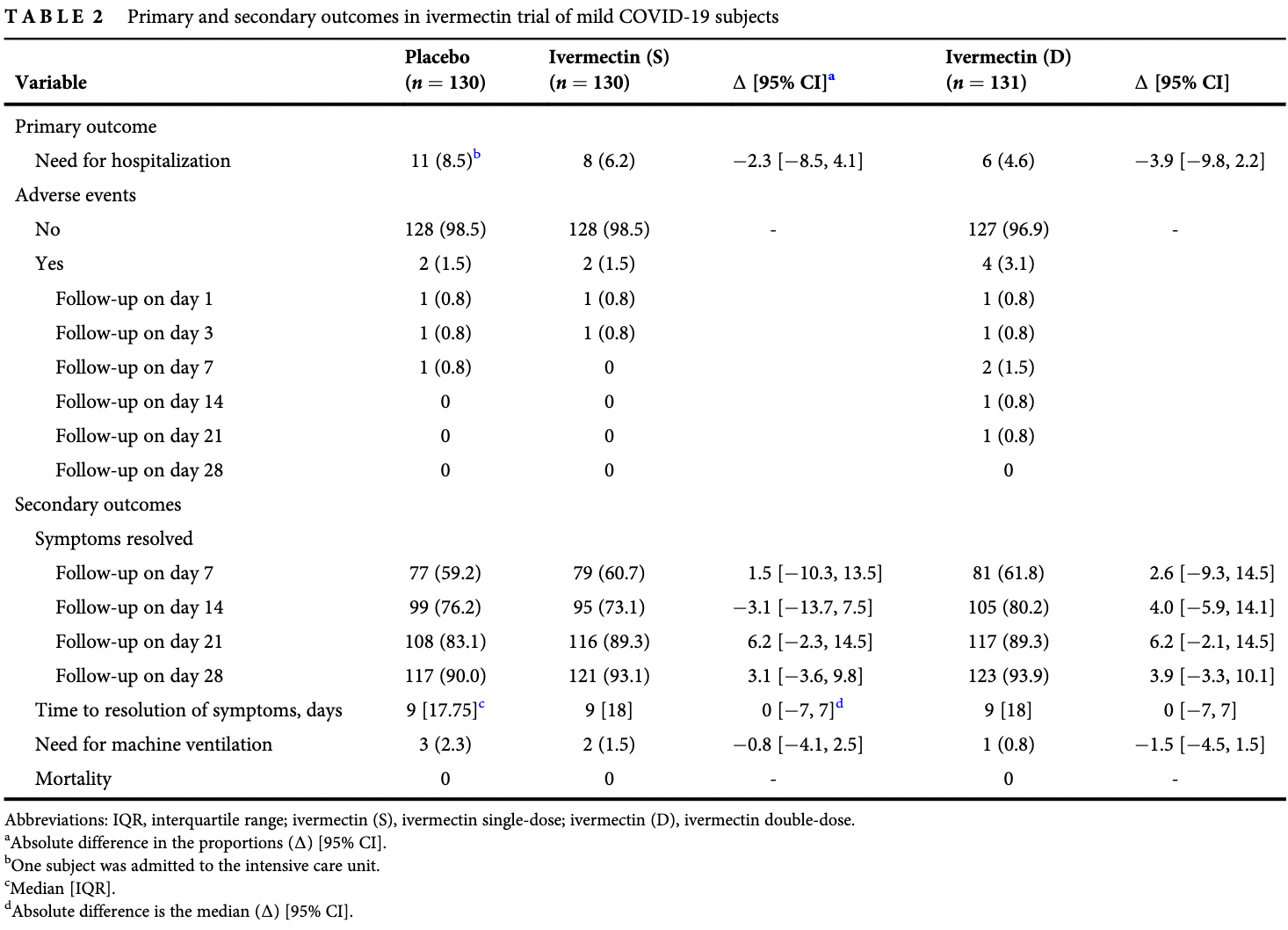
RCT with 131 24mg ivermectin, 130 12mg ivermectin, and 130 placebo patients, showing no significant differences in outcomes. Lower ventilation and hospitalization was seen with treatment, in a dose-dependent manner, but not reaching statistical significance with the small number of events.
This is the 39th of 53 COVID-19 RCTs for ivermectin, which collectively show efficacy with p=0.000000087.
This is the 88th of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
|
risk of mechanical ventilation, 66.9% lower, RR 0.33, p = 0.37, treatment 1 of 131 (0.8%), control 3 of 130 (2.3%), NNT 65, 24mg.
|
|
risk of mechanical ventilation, 33.3% lower, RR 0.67, p = 1.00, treatment 2 of 130 (1.5%), control 3 of 130 (2.3%), NNT 130, 12mg.
|
|
risk of hospitalization, 45.9% lower, RR 0.54, p = 0.22, treatment 6 of 131 (4.6%), control 11 of 130 (8.5%), NNT 26, 24mg, primary outcome.
|
|
risk of hospitalization, 27.3% lower, RR 0.73, p = 0.63, treatment 8 of 130 (6.2%), control 11 of 130 (8.5%), NNT 43, 12mg, primary outcome.
|
|
risk of no recovery, 38.9% lower, RR 0.61, p = 0.27, treatment 8 of 131 (6.1%), control 13 of 130 (10.0%), NNT 26, day 28, 24mg.
|
|
risk of no recovery, 30.8% lower, RR 0.69, p = 0.50, treatment 9 of 130 (6.9%), control 13 of 130 (10.0%), NNT 32, day 28, 12mg.
|
|
recovery time, no change, relative time 1.00, treatment 131, control 130, 24mg.
|
|
recovery time, no change, relative time 1.00, treatment 130, control 130, 12mg.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mirahmadizadeh et al., 23 Jun 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 12 authors, study period 9 April, 2021 - 20 May, 2021, dosage 24mg single dose, 12mg and 24mg arms.
Efficacy of single‐dose and double‐dose ivermectin early treatment in preventing progression to hospitalization in mild COVID‐19: A multi‐arm, parallel‐group randomized, double‐blind, placebo‐controlled trial
Respirology, doi:10.1111/resp.14318
Background and objective: Ivermectin is a known anti-parasitic agent that has been investigated as an antiviral agent against coronavirus disease 2019 (COVID-19). This study aimed to evaluate the efficacy of ivermectin in mild COVID-19 patients. Methods: In this multi-arm randomized clinical trial conducted between 9 April 2021 and 20 May 2021, a total of 393 patients with reverse transcription-PCR-confirmed COVID-19 infection and mild symptoms were enrolled. Subjects were randomized in a 1:1:1 ratio to receive single-dose ivermectin (12 mg), double-dose ivermectin (24 mg) or placebo. The primary outcome was need for hospitalization. Results: There was no significant difference in the proportion of subjects who required hospitalization between the placebo and single-dose ivermectin groups (absolute difference in the proportions: À2.3 [95% CI = À8.5, 4.1]) and between the placebo and double-dose ivermectin groups (absolute difference in the proportions: À3.9 [95% CI = À9.8, 2.2]). The odds of differences in mean change in severity score between single-dose ivermectin and placebo groups (OR difference = 1.005 [95% CI: 0.972, 1.040]; p = 0.762) and double-dose ivermectin and placebo groups (OR difference = 1.010 [95% CI: 0.974, 1.046]; p = 0.598) were not statistically significant. None of the six adverse events (including mild dermatitis, tachycardia and hypertension) were serious and required extra action. Conclusion: Single-dose and double-dose ivermectin early treatment were not superior to the placebo in preventing progression to hospitalization and improving clinical course in mild COVID-19.
CONFLICT OF INTEREST None declared.
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
How to cite this article:
References
Bloomberg, Remdesivir Averts Hospitalization in Study of High-Risk Patients
Buonfrate, Chesini, Martini, Roncaglioni, Fernandez et al., High dose ivermectin for the early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof of concept clinical trial, Int J Antimicrob Agents
Caly, Druce, Catton, Jans, Wagstaff, The FDAapproved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Caly, Wagstaff, Jans, Nuclear trafficking of proteins from RNA viruses: potential target for antivirals?, Antiviral Res, doi:10.1016/j.antiviral.2012.06.008
Chowdhury, Shahbaz, Karim, Islam, Dan et al., A comparative study on ivermectin-doxycycline and hydroxychloroquineazithromycin therapy on COVID-19 patients, EJMO
Crump, Omura, Ivermectin, 'wonder drug' from Japan: the human use perspective, Proc Jpn Acad Ser B Phys Biol Sci, doi:10.2183/pjab.87.13
Götz, Magar, Dornfeld, Giese, Pohlmann et al., Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import, Sci Rep
Kaur, Shekhar, Sharma, Sarma, Prakash et al., Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes, Pharmacol Rep, doi:10.1007/s43440-020-00195-y
Kernan, Viscoli, Makuch, Brass, Horwitz, Stratified randomization for clinical trials, J Clin Epidemiol, doi:10.1016/s0895-4356(98)00138-3
Krolewiecki, Lifschitz, Moragas, Travacio, Valentini et al., Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100959
Laing, Devaney, Ivermectinold drug, new tricks?, Trends Parasitol, doi:10.1016/j.pt.2017.02.004
Ledford, Antibody therapies could be a bridge to a coronavirus vaccinebut will the world benefit?, Nature, doi:10.1038/d41586-020-02360-y
Ledford, COVID antiviral pills: what scientists still want to know, Nature, doi:10.1038/d41586-021-03074-5
Lundberg, Pinkham, Baer, Amaya, Narayanan et al., Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan equine encephalitis virus replication, Antiviral Res, doi:10.1016/j.antiviral.2013.10.004
Mallapaty, COVID vaccines slash viral spreadbut Delta is an unknown, Nature, doi:10.1038/d41586-021-02054-z
Mastrangelo, Pezzullo, Burghgraeve, Kaptein, Pastorino et al., Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother, doi:10.1093/jac/dks147
Navarro, Camprubí, Requena-Méndez, Buonfrate, Giorli et al., Safety of high-dose ivermectin: a systematic review and metaanalysis, J Antimicrob Chemother, doi:10.1093/jac/dkz524
Opez-Medina, Hurtado, Ramirez, Martínez, Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.3071
Roy, Pattadar, Raj, Agarwal, Biswas et al., Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in eastern India, J Pharm Pharm Sci, doi:10.18433/jpps32105
Samaha, Mouawia, Fawaz, Hassan, Salami et al., Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARSCoV-2 infected subjects: a pilot clinical trial in Lebanon, Viruses, doi:10.3390/v13060989
Tay, Fraser, Chan, Moreland, Rathore et al., Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor ivermectin, Antiviral Res, doi:10.1016/j.antiviral.2013.06.002
Vallejos, Zoni, Bangher, Villamandos, Bobadilla et al., Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04813-1
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J, doi:10.1042/BJ20120150
Willyard, How antiviral pill molnupiravir shot ahead in the COVID drug hunt, Nature, doi:10.1038/d41586-021-02783-1
Yang, Atkinson, Wang, Lee, Bogoyevitch et al., The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res, doi:10.1016/j.antiviral.2020.104760
DOI record:
{
"DOI": "10.1111/resp.14318",
"ISSN": [
"1323-7799",
"1440-1843"
],
"URL": "http://dx.doi.org/10.1111/resp.14318",
"alternative-id": [
"10.1111/resp.14318"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-12-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-05-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-06-23"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2259-4984",
"affiliation": [
{
"name": "Non‐communicable Diseases Research Center Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Mirahmadizadeh",
"given": "Alireza",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-6322-7876",
"affiliation": [
{
"name": "Non‐communicable Diseases Research Center Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Semati",
"given": "Ali",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6567-5306",
"affiliation": [
{
"name": "Non‐communicable Diseases Research Center Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Heiran",
"given": "Alireza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2724-039X",
"affiliation": [
{
"name": "Communicable Diseases Control Center Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Ebrahimi",
"given": "Mostafa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9951-8341",
"affiliation": [
{
"name": "Department of Health Affairs Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Hemmati",
"given": "Abdolrasool",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1435-3996",
"affiliation": [
{
"name": "Department of Health Affairs Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Karimi",
"given": "Mohammadreza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8474-8822",
"affiliation": [
{
"name": "Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Basir",
"given": "Souzan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0199-3230",
"affiliation": [
{
"name": "Non‐communicable Diseases Research Center Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Zare",
"given": "Marjan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5516-5177",
"affiliation": [
{
"name": "Institute of Tropical Medicine, School of Medicine University of São Paulo São Paulo Brazil"
}
],
"authenticated-orcid": false,
"family": "Charlys da Costa",
"given": "Antonio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4233-9275",
"affiliation": [
{
"name": "National Zoonoses Control Department Ministry of Health and Medical Education Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Zeinali",
"given": "Mohammad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7689-9866",
"affiliation": [
{
"name": "Communicable Diseases Control Center Ministry of Health and Medical Education Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Sargolzaee",
"given": "Maryam",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5313-1352",
"affiliation": [
{
"name": "Department of Family Medicine and Infectious Diseases Shiraz University of Medical Sciences Shiraz Iran"
}
],
"authenticated-orcid": false,
"family": "Eilami",
"given": "Owrang",
"sequence": "additional"
}
],
"container-title": "Respirology",
"container-title-short": "Respirology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
24
]
],
"date-time": "2022-06-24T00:59:40Z",
"timestamp": 1656032380000
},
"deposited": {
"date-parts": [
[
2022,
6,
24
]
],
"date-time": "2022-06-24T01:00:00Z",
"timestamp": 1656032400000
},
"funder": [
{
"DOI": "10.13039/501100004320",
"award": [
"99‐7850"
],
"doi-asserted-by": "publisher",
"name": "Shiraz University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2022,
6,
24
]
],
"date-time": "2022-06-24T01:41:33Z",
"timestamp": 1656034893495
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
6,
23
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
23
]
],
"date-time": "2022-06-23T00:00:00Z",
"timestamp": 1655942400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
23
]
],
"date-time": "2022-06-23T00:00:00Z",
"timestamp": 1655942400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/resp.14318",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/resp.14318",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/resp.14318",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
6,
23
]
]
},
"published-online": {
"date-parts": [
[
2022,
6,
23
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "#cr-split#-e_1_2_10_2_1.1",
"unstructured": "World Health Organization.COVID‐19: Clinical Care. Therapeutics and COVID‐19: Living Guideline. [updated 2021 Sep 25"
},
{
"key": "#cr-split#-e_1_2_10_2_1.2",
"unstructured": "cited 2021 Sep 28]. Available from:https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2"
},
{
"DOI": "10.1038/d41586-020-02360-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1038/d41586-021-02783-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1038/d41586-021-03074-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"key": "e_1_2_10_6_1",
"unstructured": "Bloomberg.Remdesivir Averts Hospitalization in Study of High‐Risk Patients.2021Sep 22 [cited 2021 Nov 27]. Available from:https://www.bloomberg.com/news/articles/2021-09-22/remdesivir-averts-hospitalization-in-study-of-high-risk-patients"
},
{
"DOI": "10.1038/d41586-021-02054-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.2183/pjab.87.13",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"key": "e_1_2_10_9_1",
"unstructured": "World Health Organization.World Health Organization Model List of Essential Medicines: 21st List 2019.Geneva:World Health Organization;2019[cited 2021 Sep 19]. Available from:https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf"
},
{
"DOI": "10.1016/j.pt.2017.02.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1038/srep23138",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1016/j.antiviral.2013.10.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1042/BJ20120150",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1016/j.antiviral.2012.06.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1093/jac/dks147",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.3390/v13060989",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1001/jama.2021.3071",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1186/s13063-020-04813-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1093/jac/dkz524",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1007/s43440-020-00195-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"key": "#cr-split#-e_1_2_10_24_1.1",
"unstructured": "World Health Organization.COVID‐19 Therapeutic Trial Synopsis Draft. [updated 2020 Feb 18"
},
{
"key": "#cr-split#-e_1_2_10_24_1.2",
"unstructured": "cited 2021 Sep 19]. Available from:https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis"
},
{
"article-title": "A comparative study on ivermectin‐doxycycline and hydroxychloroquine‐azithromycin therapy on COVID‐19 patients",
"author": "Chowdhury A",
"first-page": "63",
"issue": "1",
"journal-title": "EJMO",
"key": "e_1_2_10_25_1",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/s0895-4356(98)00138-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.18433/jpps32105",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1016/j.ijantimicag.2021.106516",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1016/j.eclinm.2021.100959",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"key": "#cr-split#-e_1_2_10_30_1.1",
"unstructured": "World Health Organization.WHO Advises that Ivermectin Only Be Used to Treat COVID‐19 Within Clinical Trials. [updated 2021 Mar 31"
},
{
"key": "#cr-split#-e_1_2_10_30_1.2",
"unstructured": "cited 2021 Sep 19]. Available from:https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials"
},
{
"key": "e_1_2_10_31_1",
"unstructured": "Centers for Disease Control and Prevention (CDC).Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID‐19. [updated 2021 Aug 26; cited 2021 Sep 19]. Available from:https://emergency.cdc.gov/han/2021/han00449.asp"
},
{
"key": "#cr-split#-e_1_2_10_32_1.1",
"unstructured": "Google News.Coronavirus (COVID‐19). [updated 2021 Sep 27"
},
{
"key": "#cr-split#-e_1_2_10_32_1.2",
"unstructured": "cited 2021 Oct 2]. Available from:https://news.google.com/covid19/map?hl=fa&state=7&mid=%2Fm%2F03shp"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/resp.14318"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Efficacy of single‐dose and double‐dose ivermectin early treatment in preventing progression to hospitalization in mild\n <scp>COVID</scp>\n ‐19: A multi‐arm, parallel‐group randomized, double‐blind, placebo‐controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
