Efficacy of Nirmatrelvir/Ritonavir (Paxlovid) for COVID-19 in Vaccinated Patients With Inflammatory Bowel Disease
et al., American Journal of Gastroenterology, doi:10.14309/01.ajg.0001082744.48729.45, Dec 2024
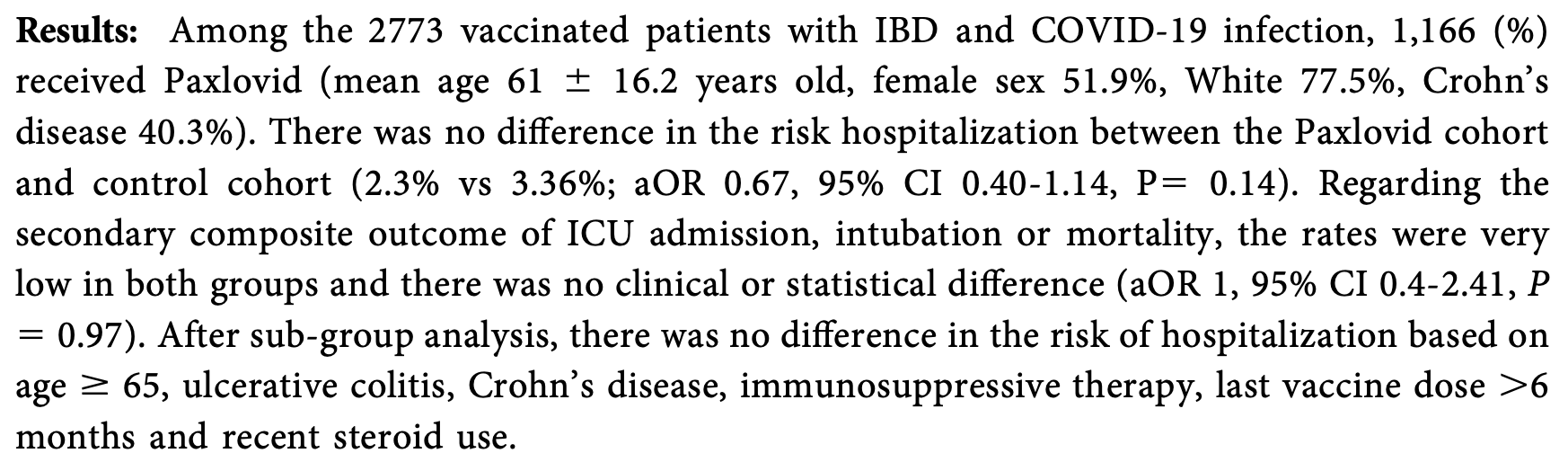
PSM retrospective 2,773 IBD patients showing no significant difference in hospitalization or the composite outcome of ICU admission, intubation, or mortality with paxlovid treatment. Authors do not specify exclusion of contraindicated patients from the control group which may result in overestimating efficacy.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments18.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of progression, no change, OR 1.00, p = 0.97, treatment 1,166, control 1,607, ICU admission, intubation, or mortality, RR approximated with OR.
|
|
risk of hospitalization, 33.0% lower, OR 0.67, p = 0.14, treatment 1,166, control 1,607, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Mikhail et al., 12 Dec 2024, retrospective, USA, peer-reviewed, 8 authors.
Efficacy of Nirmatrelvir/Ritonavir (Paxlovid) for COVID-19 in Vaccinated Patients With Inflammatory Bowel Disease
Background: Obefazimod (Obe) is an oral, once-daily (od) small molecule that enhances expression of microRNA-124 and is in phase 3 trials for moderately to severely active ulcerative colitis (UC). Obe showed efficacy and safety at week 8 in a placebo-controlled Phase 2b induction trial and a subsequent open-label maintenance (OLM) study. This post hoc analysis reports corticosteroid (CS)-free efficacy and safety at weeks 48 and 96 in patients receiving concomitant CS at induction baseline. Methods: In the Phase 2b induction trial, patients received placebo or Obe (25 mg, 50 mg, or 100 mg od) and could enter the optional 96-week OLM study with Obe 50 mg, regardless of clinical response. Patients were monitored monthly for safety and efficacy. At induction trial entry, patients could use concomitant CS if on a stable dose for $2 weeks before screening. CS tapering was recommended but not required at the OLM study baseline. CS-free status was defined as CS withdrawal $12 weeks before assessment. CS-free outcomes, including clinical remission (CR), endoscopic improvement (EI), and endoscopic remission (ER), were evaluated at weeks 48 and 96, along with safety. Results: At induction baseline, 115 patients (out of 252, 46%) received CS. Among patients with CS at induction baseline, CR rates were 56% at week 48 and 52% at week 96. CS-free CR was attained by 37% (43/115) at week 48 and 35% at week 96 within the same cohort. Among the 21 patients that were in CR but did not meet the definition of CS-free at week 48, the Principal Investigator (PI) did not attempt the optional CS tapering for 13 pts. An additional 4 patients were CS free, but for less than 12 weeks before the week 48 endpoint. Among the 20 patients that were in CR but did not meet the definition of CS-free at week 96, the PI did not attempt the optional CS tapering for 8 patients. An additional 7 patients were CS free, but for less than 12 weeks before the week 96 endpoint. EI was achieved by 64% at week 48 and 58% at week 96, with CS-free EI achieved by 44% of patients at week 48 and 39% at week 96. ER was achieved by 33% at week 48 and 31% at week 96, with CS-free ER achieved by 24% of patients at week 48 and 21% at week 96. Among patients with CS at induction baseline, 50% and 39% of patients had FCP , 150 mg/g at weeks 48 and 96, respectively. CS-free patients with FCP , 150 mg/g were 32% at week 48 and 28% at week 96. Overall, the safety profile within this cohort was consistent with the overall study population. Conclusions: Among patients receiving concomitant CS at induction baseline, meaningful proportions achieved CR, EI, and ER at weeks 48 and 96 in the OLM. Notably, a substantial proportion of patients met CS-free efficacy endpoints despite optional CS tapering. Concomitant CS use at induction baseline had no notable effect on the safety profile of Obe.
DOI record:
{
"DOI": "10.14309/01.ajg.0001082744.48729.45",
"ISSN": [
"0002-9270",
"1572-0241"
],
"URL": "http://dx.doi.org/10.14309/01.ajg.0001082744.48729.45",
"author": [
{
"affiliation": [
{
"name": "Mayo Clinic, Jacksonville, Florida, United States;"
}
],
"family": "Mikhail",
"given": "Inas",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Allegheny Health Network, Pittsburgh, Pennsylvania, United States;"
}
],
"family": "Desai",
"given": "Aakash",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Mayo Clinic;"
}
],
"family": "Crosby",
"given": "Sheena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Gastroenterology and Hepatology, Mayo Clinic;"
}
],
"family": "Hashash",
"given": "Jana G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Wisconsin School of Pharmacy, Madison, Wisconsin, United States;"
}
],
"family": "Hayney",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Wisconsin–Madison;"
}
],
"family": "Caldera",
"given": "Freddy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Allegheny Health Network, Pittsburgh, Pennsylvania, United States;"
}
],
"family": "Kochhar",
"given": "Gursimran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IBD Center, Mayo Clinic Florida."
}
],
"family": "Farraye",
"given": "Francis A. A.",
"sequence": "additional"
}
],
"container-title": "American Journal of Gastroenterology",
"container-title-short": "Am J Gastroenterol",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"lww.com",
"ovid.com"
]
},
"created": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T12:02:14Z",
"timestamp": 1733745734000
},
"deposited": {
"date-parts": [
[
2024,
12,
9
]
],
"date-time": "2024-12-09T12:02:14Z",
"timestamp": 1733745734000
},
"indexed": {
"date-parts": [
[
2024,
12,
10
]
],
"date-time": "2024-12-10T05:12:24Z",
"timestamp": 1733807544303,
"version": "3.30.1"
},
"is-referenced-by-count": 0,
"issue": "12S",
"issued": {
"date-parts": [
[
2024,
12
]
]
},
"journal-issue": {
"issue": "12S",
"published-print": {
"date-parts": [
[
2024
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.14309/01.ajg.0001082744.48729.45",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "S15-S15",
"prefix": "10.14309",
"published": {
"date-parts": [
[
2024,
12
]
]
},
"published-print": {
"date-parts": [
[
2024,
12
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.14309/01.ajg.0001082744.48729.45"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "S53 Efficacy of Nirmatrelvir/Ritonavir (Paxlovid) for COVID-19 in Vaccinated Patients With Inflammatory Bowel Disease",
"type": "journal-article",
"update-policy": "https://doi.org/10.1097/lww.0000000000001000",
"volume": "119"
}