Free Zinc as a Predictive Marker for COVID-19 Mortality Risk
et al., Nutrients, doi:10.3390/nu14071407, Mar 2022
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000028 from 47 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
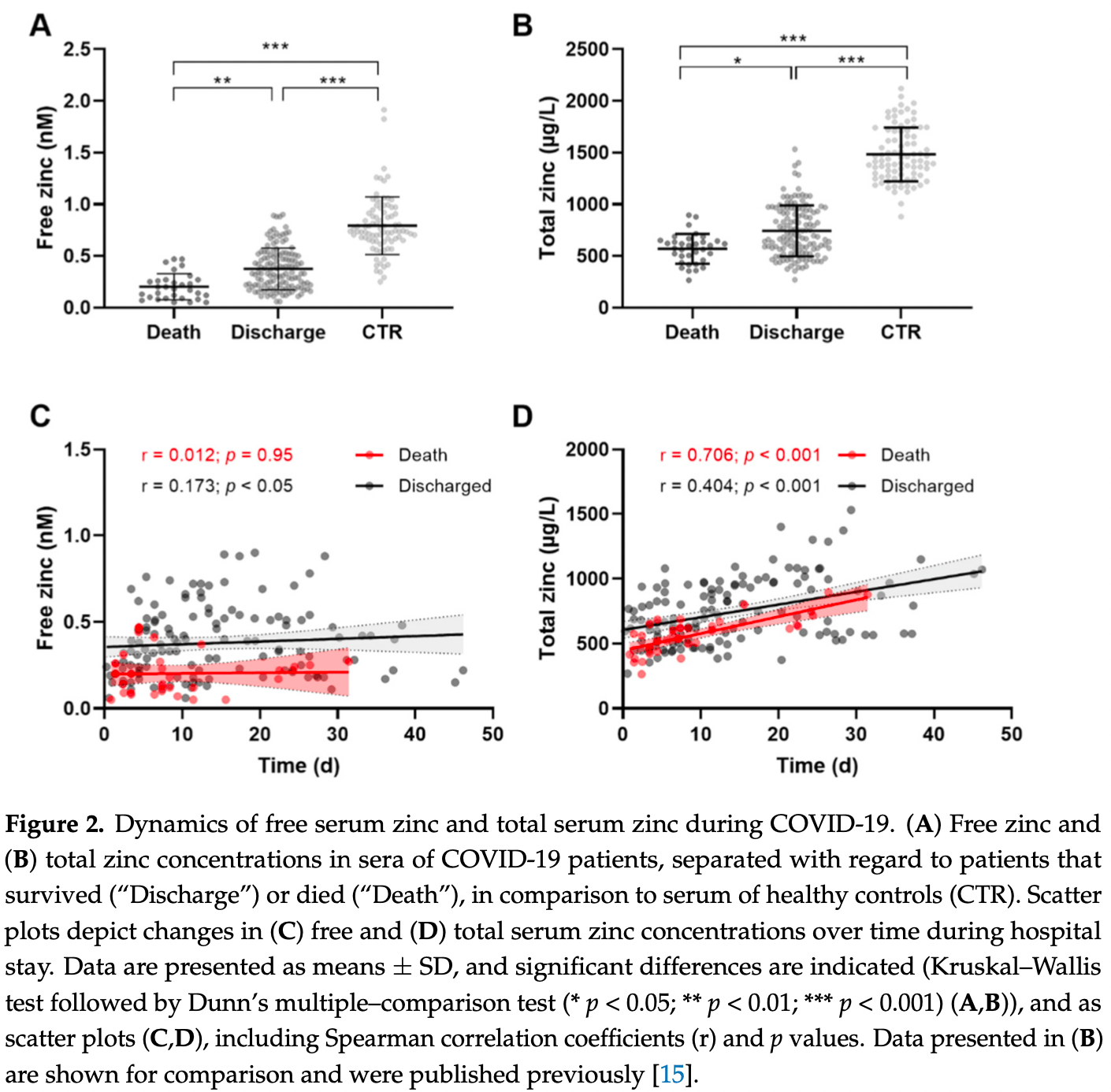
Analysis of 33 COVID-19 patients and 86 control patients in Germany, showing lower free serum zinc levels associated with COVID-19 and mortality.
Maares et al., 28 Mar 2022, retrospective, Germany, peer-reviewed, 9 authors.
Free Zinc as a Predictive Marker for COVID-19 Mortality Risk
Nutrients, doi:10.3390/nu14071407
Free zinc is considered to be the exchangeable and biological active form of zinc in serum, and is discussed to be a suitable biomarker for alterations in body zinc homeostasis and related diseases. Given that coronavirus disease 2019 (COVID-19) is characterized by a marked decrease in total serum zinc, and clinical data indicate that zinc status impacts the susceptibility and severity of the infection, we hypothesized that free zinc in serum might be altered in response to SARS-CoV-2 infection and may reflect disease severity. To test this hypothesis, free zinc concentrations in serum samples of survivors and nonsurvivors of COVID-19 were analyzed by fluorometric microassay. Similar to the reported total serum zinc deficit measured by total reflection X-ray fluorescence, free serum zinc in COVID-19 patients was considerably lower than that in control subjects, and surviving patients displayed significantly higher levels of free zinc than those of nonsurvivors (mean ± SD; 0.4 ± 0.2 nM vs. 0.2 ± 0.1 nM; p = 0.0004). In contrast to recovering total zinc concentrations (r = 0.706, p < 0.001) or the declining copper-zinc ratio (r = −0.646; p < 0.001), free zinc concentrations remained unaltered with time in COVID-19 nonsurvivors. Free serum zinc concentrations were particularly low in male as compared to female patients (mean ± SD; 0.4 ± 0.2 nM vs. 0.2 ± 0.1 nM; p = 0.0003). This is of particular interest, as the male sex is described as a risk factor for severe COVID-19. Overall, results indicate that depressed free serum zinc levels are associated with increased risk of death in COVID-19, suggesting that free zinc may serve as a novel prognostic marker for the severity and course of COVID-19.
References
Alexander, Tinkov, Strand, Alehagen, Skalny et al., Early nutritional interventions with zinc, selenium and vitamin d for raising anti-viral resistance against progressive COVID-19, Nutrients, doi:10.3390/nu12082358
Alker, Haase, Zinc and sepsis, Nutrients, doi:10.3390/nu10080976
Alker, Schwerdtle, Schomburg, Haase, A zinpyr-1-based fluorimetric microassay for free zinc in human serum, Int. J. Mol. Sci, doi:10.3390/ijms20164006
Anuk, Polat, Akdas, Erol, Tanacan et al., The relation between trace element status (zinc, copper, magnesium) and clinical outcomes in COVID-19 infection during pregnancy, Biol. Trace Elem. Res, doi:10.1007/s12011-020-02496-y
Aziz, Fatima, Assaly, Elevated interleukin-6 and severe COVID-19: A meta-analysis, J. Med. Virol
Aziz, Fatima, Lee-Smith, Assaly, The association of low serum albumin level with severe COVID-19: A systematic review and meta-analysis, Crit. Care, doi:10.1186/s13054-020-02995-3
Bagher Pour, Yahyavi, Karimi, Khamaneh, Milani et al., Serum trace elements levels and clinical outcomes among iranian COVID-19 patients, Int. J. Infect. Dis, doi:10.1016/j.ijid.2021.08.053
Bechmann, Barthel, Schedl, Herzig, Varga et al., Sexual dimorphism in COVID-19: Potential clinical and public health implications, Lancet Diabetes Endocrinol
Besecker, Exline, Hollyfield, Phillips, Disilvestro et al., A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission, Am. J. Clin. Nutr, doi:10.3945/ajcn.110.008417
Bornhorst, Kipp, Haase, Meyer, Schwerdtle, The crux of inept biomarkers for risks and benefits of trace elements, TrAC Trends Anal. Chem, doi:10.1016/j.trac.2017.11.007
Broadley, Edward, Ander, Michael, Scott et al., Dietary calcium and zinc deficiency risks are decreasing but remain prevalent, Sci. Rep
Brown, Rivera, Bhutta, Gibson, King et al., International zinc nutrition consultative group (izincg) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control, Food Nutr. Bull
Burdette, Walkup, Spingler, Tsien, Lippard, Fluorescent sensors for Zn 2+ based on a fluorescein platform: Synthesis, properties and intracellular distribution, J. Am. Chem. Soc, doi:10.1021/ja010059l
Cheng, Bar, Tako, Zinc status index (zsi) for quantification of zinc physiological status, Nutrients, doi:10.3390/nu13103399
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-ncov) by real-time rt-pcr, EuroSurveillance, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Fukada, Yamasaki, Nishida, Murakami, Hirano, Zinc homeostasis and signaling in health and diseases: Zinc signaling, J. Biol. Inorg. Chem, doi:10.1007/s00775-011-0797-4
Galmés, Serra, Palou, Current state of evidence: Influence of nutritional and nutrigenetic factors on immunity in the COVID-19 pandemic framework, Nutrients, doi:10.3390/nu12092738
Gonçalves, Gonçalves, Guarnieri, Risegato, Guimarães et al., Association between low zinc levels and severity of acute respiratory distress syndrome by new coronavirus sars-cov-2, Nutr. Clin. Pract, doi:10.1002/ncp.10612
Grynkiewicz, Poenie, Tsien, A new generation of Ca 2+ indicators with greatly improved fluorescence properties, J. Biol. Chem, doi:10.1016/S0021-9258(19)83641-4
Haase, Hebel, Engelhardt, Rink, The biochemical effects of extracellular Zn(2+) and other metal ions are severely affected by their speciation in cell culture media, Met. Integr. Biometal Sci, doi:10.1039/C4MT00206G
Hackler, Heller, Sun, Schwarzer, Diegmann et al., Relation of serum copper status to survival in COVID-19, Nutrients, doi:10.3390/nu13061898
Hackler, Wisniewska, Greifenstein-Wiehe, Minich, Cremer et al., Copper and selenium status as biomarkers of neonatal infections, J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem, doi:10.1016/j.jtemb.2019.126437
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat. Rev. Microbiol, doi:10.1038/s41579-021-00573-0
Heller, Sun, Hackler, Seelig, Seibert et al., Prediction of survival odds in COVID-19 by zinc, age and selenoprotein p as composite biomarker, Redox Biol
Hoeger, Simon, Beeker, Marx, Haase et al., Persistent low serum zinc is associated with recurrent sepsis in critically ill patients -a pilot study, PLoS ONE, doi:10.1371/journal.pone.0176069
Hoeger, Simon, Doemming, Thiele, Marx et al., Alterations in zinc binding capacity, free zinc levels and total serum zinc in a porcine model of sepsis, BioMetals, doi:10.1007/s10534-015-9858-4
Hybsier, Schulz, Wu, Demuth, Minich et al., Sex-specific and inter-individual differences in biomarkers of selenium status identified by a calibrated elisa for selenoprotein p, Redox Biol, doi:10.1016/j.redox.2016.12.025
Jothimani, Kailasam, Danielraj, Nallathambi, Ramachandran et al., COVID-19: Poor outcomes in patients with zinc deficiency, Int. J. Infect. Dis
Kehl-Fie, Skaar, Nutritional immunity beyond iron: A role for manganese and zinc, Curr. Opin. Chem. Biol, doi:10.1016/j.cbpa.2009.11.008
Keleş, Yılmaz Çiftdo Gan, Çolak, Kara Aksay, Üstündag et al., Serum zinc levels in pediatric patients with COVID-19, Eur. J. Pediatr, doi:10.1007/s00431-021-04348-w
Kocak, Ozgeris, Parlak, Kadıoglu, Yuce et al., Evaluation of serum trace element levels and biochemical parameters of COVID-19 patients according to disease severity, Biol. Trace Elem. Res, doi:10.1007/s12011-021-02946-1
Konz, Santoro, Goulet, Bazzocchi, Battista et al., Sex-specific associations of blood-based nutrient profiling with body composition in the elderly, Front. Physiol, doi:10.3389/fphys.2018.01935
Laine, Tuomainen, Salonen, Virtanen, Serum copper-to-zinc-ratio and risk of incident infection in men: The kuopio ischaemic heart disease risk factor study, Eur. J. Epidemiol, doi:10.1007/s10654-020-00644-1
Laing, Petrovic, Lachat, De Boevre, Klingenberg et al., Course and survival of COVID-19 patients with comorbidities in relation to the trace element status at hospital admission, Nutrients, doi:10.3390/nu13103304
Li, To, Biomarkers for severe COVID-19, EBioMedicine, doi:10.1016/j.ebiom.2021.103405
Liuzzi, Lichten, Rivera, Blanchard, Aydemir et al., Interleukin-6 regulates the zinc transporter zip14 in liver and contributes to the hypozincemia of the acute-phase response, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0502257102
Maares, Haase, A guide to human zinc absorption: General overview and recent advances of in vitro intestinal models, Nutrients, doi:10.3390/nu12030762
Maggini, Wintergerst, Beveridge, Hornig, Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses, Br. J. Nutr, doi:10.1017/S0007114507832971
Malavolta, Giacconi, Piacenza, Santarelli, Cipriano et al., Plasma copper/zinc ratio: An inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population, Biogerontology, doi:10.1007/s10522-009-9251-1
Malavolta, Piacenza, Basso, Giacconi, Costarelli et al., Serum copper to zinc ratio: Relationship with aging and health status, Mech. Ageing Dev, doi:10.1016/j.mad.2015.01.004
Mayor-Ibarguren, Busca-Arenzana, Robles-Marhuenda, A hypothesis for the possible role of zinc in the immunological pathways related to COVID-19 infection, Front. Immunol, doi:10.3389/fimmu.2020.01736
Mertens, Lowes, Webster, Talib, Hall et al., Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation, Br. J. Anaesth, doi:10.1093/bja/aev073
Moghaddam, Heller, Sun, Seelig, Cherkezov et al., Selenium deficiency is associated with mortality risk from COVID-19, Nutrients, doi:10.3390/nu12072098
Notz, Herrmann, Schlesinger, Helmer, Sudowe et al., Clinical significance of micronutrient supplementation in critically ill COVID-19 patients with severe ards, Nutrients
Patel, Chinni, El-Khoury, Perera, Neto et al., A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients, J. Med. Virol, doi:10.1002/jmv.26895
Peckham, De Gruijter, Raine, Radziszewska, Ciurtin et al., Male sex identified by global COVID-19 meta-analysis as a risk factor for death and itu admission, Nat. Commun
Pvsn, Tomo, Purohit, Sankanagoudar, Charan et al., Comparative analysis of serum zinc, copper and magnesium level and their relations in association with severity and mortality in SARS-CoV-2 patients, Biol. Trace Elem. Res, doi:10.1007/s12011-022-03124-7
Read, O'connor, Suppiah, Ahlenstiel, Obeid et al., Zinc is a potent and specific inhibitor of ifn-λ3 signalling, Nat. Commun, doi:10.1038/ncomms15245
Read, Obeid, Ahlenstiel, Ahlenstiel, The role of zinc in antiviral immunity, Adv. Nutr, doi:10.1093/advances/nmz013
Samprathi, Jayashree, Biomarkers in COVID-19: An up-to-date review, Front. Pediatr, doi:10.3389/fped.2020.607647
Skalny, Timashev, Aschner, Aaseth, Chernova et al., Serum zinc, copper, and other biometals are associated with COVID-19 severity markers, Metabolites, doi:10.3390/metabo11040244
Tsoukalas, Sarandi, Micronutrient deficiencies in patients with COVID-19: How metabolomics can contribute to their prevention and replenishment, BMJ Nutr. Prev. Health, doi:10.1136/bmjnph-2020-000169
Velthuis, Van Den Worm, Sims, Baric, Snijder et al., Zn 2+ inhibits coronavirus and arterivirus rna polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture, PLoS Pathog, doi:10.1371/journal.ppat.1001176
Wandt, Winkelbeiner, Lossow, Kopp, Schwarz et al., Ageing-associated effects of a long-term dietary modulation of four trace elements in mice, Redox Biol
Wang, Fan, Wei, Wang, Yu et al., Integrated multi-omics uncovers reliable potential biomarkers and adverse effects of zinc deficiency, Clin. Nutr, doi:10.1016/j.clnu.2021.03.019
Wang, Yang, Wang, Li, Ning et al., Calcium-deficiency assessment and biomarker identification by an integrated urinary metabonomics analysis, BMC Med, doi:10.1186/1741-7015-11-86
Wessels, Maywald, Rink, Zinc as a gatekeeper of immune function, Nutrients, doi:10.3390/nu9121286
Wessels, Rolles, Rink, The potential impact of zinc supplementation on COVID-19 pathogenesis, Front. Immunol, doi:10.3389/fimmu.2020.01712
DOI record:
{
"DOI": "10.3390/nu14071407",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu14071407",
"abstract": "<jats:p>Free zinc is considered to be the exchangeable and biological active form of zinc in serum, and is discussed to be a suitable biomarker for alterations in body zinc homeostasis and related diseases. Given that coronavirus disease 2019 (COVID-19) is characterized by a marked decrease in total serum zinc, and clinical data indicate that zinc status impacts the susceptibility and severity of the infection, we hypothesized that free zinc in serum might be altered in response to SARS-CoV-2 infection and may reflect disease severity. To test this hypothesis, free zinc concentrations in serum samples of survivors and nonsurvivors of COVID-19 were analyzed by fluorometric microassay. Similar to the reported total serum zinc deficit measured by total reflection X-ray fluorescence, free serum zinc in COVID-19 patients was considerably lower than that in control subjects, and surviving patients displayed significantly higher levels of free zinc than those of nonsurvivors (mean ± SD; 0.4 ± 0.2 nM vs. 0.2 ± 0.1 nM; p = 0.0004). In contrast to recovering total zinc concentrations (r = 0.706, p < 0.001) or the declining copper–zinc ratio (r = −0.646; p < 0.001), free zinc concentrations remained unaltered with time in COVID-19 nonsurvivors. Free serum zinc concentrations were particularly low in male as compared to female patients (mean ± SD; 0.4 ± 0.2 nM vs. 0.2 ± 0.1 nM; p = 0.0003). This is of particular interest, as the male sex is described as a risk factor for severe COVID-19. Overall, results indicate that depressed free serum zinc levels are associated with increased risk of death in COVID-19, suggesting that free zinc may serve as a novel prognostic marker for the severity and course of COVID-19.</jats:p>",
"alternative-id": [
"nu14071407"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9140-2448",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maares",
"given": "Maria",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9781-1680",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hackler",
"given": "Julian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haupt",
"given": "Alessia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8006-9742",
"affiliation": [],
"authenticated-orcid": false,
"family": "Heller",
"given": "Raban Arved",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bachmann",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diegmann",
"given": "Joachim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moghaddam",
"given": "Arash",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9445-1555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schomburg",
"given": "Lutz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1622-8718",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haase",
"given": "Hajo",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
30
]
],
"date-time": "2022-03-30T01:41:30Z",
"timestamp": 1648604490000
},
"deposited": {
"date-parts": [
[
2022,
3,
30
]
],
"date-time": "2022-03-30T02:38:10Z",
"timestamp": 1648607890000
},
"funder": [
{
"DOI": "10.13039/501100001659",
"award": [
"FOR 2558, HA 4318/4-2, Scho 849/6-2, CRC/TR 296"
],
"doi-asserted-by": "publisher",
"name": "Deutsche Forschungsgemeinschaft"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
13
]
],
"date-time": "2024-05-13T07:54:58Z",
"timestamp": 1715586898530
},
"is-referenced-by-count": 21,
"issue": "7",
"issued": {
"date-parts": [
[
2022,
3,
28
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
28
]
],
"date-time": "2022-03-28T00:00:00Z",
"timestamp": 1648425600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/14/7/1407/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1407",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
28
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.3389/fped.2020.607647",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/j.ebiom.2021.103405",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/S2213-8587(21)00346-6",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.3390/nu12092738",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.3390/nu13103304",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.3390/nu13062113",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.3390/nu12082358",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.3389/fimmu.2020.01712",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1093/advances/nmz013",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.ijid.2020.09.014",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.ijid.2021.08.053",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.3390/metabo11040244",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.redox.2020.101764",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1007/s12011-020-02496-y",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1002/ncp.10612",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1007/s12011-021-02946-1",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1002/jmv.26895",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1007/s00431-021-04348-w",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1007/s12011-022-03124-7",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.3390/nu12030762",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3390/nu9121286",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1038/srep10974",
"article-title": "Dietary calcium and zinc deficiency risks are decreasing but remain prevalent",
"author": "Broadley",
"doi-asserted-by": "crossref",
"first-page": "10974",
"journal-title": "Sci. Rep.",
"key": "ref24",
"volume": "5",
"year": "2015"
},
{
"article-title": "International zinc nutrition consultative group (izincg) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control",
"author": "Brown",
"first-page": "S99",
"journal-title": "Food Nutr. Bull.",
"key": "ref25",
"volume": "25",
"year": "2004"
},
{
"DOI": "10.1039/C4MT00206G",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.3390/ijms20164006",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1007/s00775-011-0797-4",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1016/j.trac.2017.11.007",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1016/S0021-9258(19)83641-4",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1021/ja010059l",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.3390/nu12072098",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3390/nu13061898",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/j.jtemb.2019.126437",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1038/ncomms15245",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1186/s13054-020-02995-3",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1007/s10534-015-9858-4",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.3390/nu13103399",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1136/bmjnph-2020-000169",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1186/1741-7015-11-86",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.clnu.2021.03.019",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1017/S0007114507832971",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.3945/ajcn.110.008417",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1371/journal.pone.0176069",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1073/pnas.0502257102",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.cbpa.2009.11.008",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.3390/nu10080976",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1002/jmv.25948",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.3389/fimmu.2020.01736",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.3389/fphys.2018.01935",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1016/j.redox.2016.12.025",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1016/j.redox.2021.102083",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1038/s41467-020-19741-6",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1093/bja/aev073",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1016/j.mad.2015.01.004",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1007/s10522-009-9251-1",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1007/s10654-020-00644-1",
"doi-asserted-by": "publisher",
"key": "ref58"
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/14/7/1407"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Free Zinc as a Predictive Marker for COVID-19 Mortality Risk",
"type": "journal-article",
"volume": "14"
}
