Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study
et al., Infection, doi:10.1007/s15010-022-01914-8, Sep 2022
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 218 COVID+ lung transplant patients in Germany, showing no significant difference in severe cases with early sotrovimab use.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
|
risk of severe case, 20.2% higher, RR 1.20, p = 0.79, treatment 21 of 125 (16.8%), control 13 of 93 (14.0%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Kneidinger et al., 9 Sep 2022, retrospective, Germany, peer-reviewed, 11 authors, study period 1 January, 2022 - 20 March, 2022, lung transplant patients.
Contact: nikolaus.kneidinger@med.uni-muenchen.de.
Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study
Infection, doi:10.1007/s15010-022-01914-8
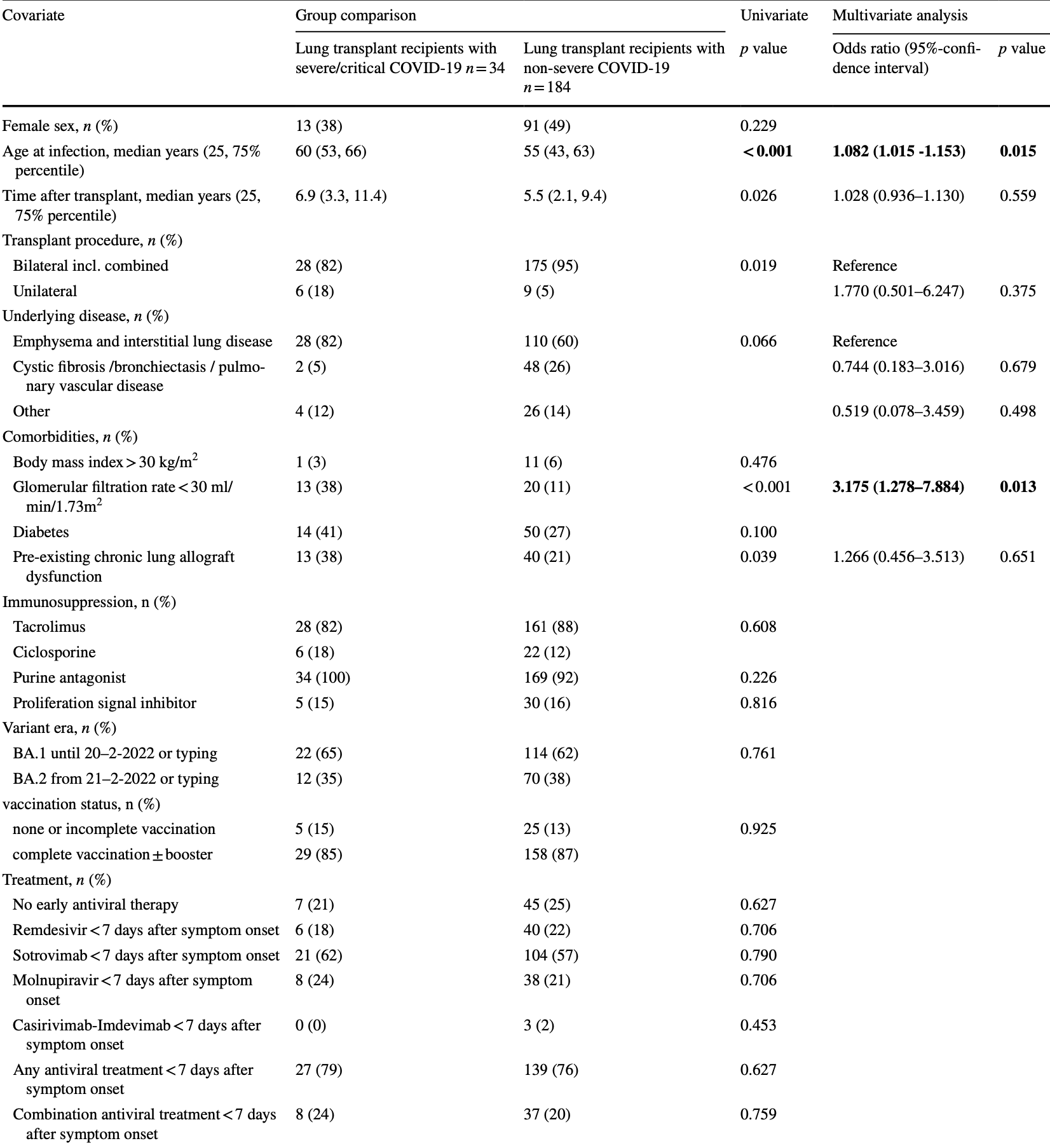
Purpose Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is currently the major threat for immunocompromised individuals. The course of COVID-19 in lung transplant recipients in the Omicron era remains unknown. The aim of the study was to assess outcome and associated factors in lung transplant recipients in a German-wide multicenter approach. Methods All affected individuals from January 1st to March 20th, 2022 from 8 German centers during the Omicron wave were collected. Baseline characteristics and antiviral measures were associated with outcome. Results Of 218 patients with PCR-proven SARS-CoV-2 infection 166 patients (76%) received any early (< 7 days) antiviral therapy median 2 (interquartile range 1-4) days after symptom onset. Most patients received sotrovimab (57%), followed by remdesivir (21%) and molnupiravir (21%). An early combination therapy was applied in 45 patients (21%). Thirty-four patients (16%) developed a severe or critical disease severity according to the WHO scale. In total, 14 patients (6.4%) died subsequently associated with COVID-19. Neither vaccination and antibody status, nor applied treatments were associated with outcome. Only age and glomerular filtration rate < 30 ml/min/1.73m 2 were independent risk factors for a severe or critical COVID-19. Conclusion COVID-19 due to Omicron remains an important threat for lung transplant recipients. In particular, elderly patients and patients with impaired kidney function are at risk for worse outcome. Prophylaxis and therapy in highly immunocompromised individuals need further improvement.
Author contributions NK and JG designed and directed the project. All authors contributed to the data collection. NK and JG performed the analysis and drafted the manuscript. All authors were involved in interpretation of results reviewed the manuscript. Funding Open Access funding enabled and organized by Projekt DEAL. Not applicable.
Declarations Competing interests The authors declare no competing interests.
Conflict of interest The authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical approval The study was approved by the central institutional ethics committee (LMU Munich, Germany; project number 22-0078). Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the..
References
Aversa, Benvenuto, Anderson, COVID-19 in lung transplant recipients: a single center case series from New York City, Am J Transplant, doi:10.1111/ajt.16241
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bouzid, Visseaux, Kassasseya, Comparison of patients infected with delta versus omicron COVID-19 variants presenting to paris emergency departments: a retrospective cohort study, Ann Intern Med, doi:10.7326/M22-0308
Coll, Fernández-Ruiz, Sánchez-Álvarez, COVID-19 in transplant recipients: the Spanish experience, Am J Transplant, doi:10.1111/ajt.16369
Fall, Eldesouki, Sachithanandham, variant delta with omicron: unprecedented spike in COVID-19 cases associated with fewer admissions and comparable upper respiratory viral loads, doi:10.1101/2022.01.26.22269927
Gleeson, Martin, Thomson, Kidney transplant recipients and omicron: outcomes, effect of vaccines and the efficacy and safety of novel treatments, medRxiv, doi:10.1101/2022.05.03.22274524
Gottlieb, Kolditz, Gade, Welte, Kneidinger, Benefit of monoclonal antibodies in early treatment of COVID-19 after lung transplantation-a retrospective analysis in two centers, Eur Respir J, doi:10.1183/13993003.00124-2022
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Gupta, Gonzalez-Rojas, Juarez, Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Hadi, Naqvi, Kupec, Sofka, Sarwari, Outcomes of COVID-19 in solid organ transplant recipients: a propensitymatched analysis of a large research network, Transplantation, doi:10.1097/TP.0000000000003670
Havlin, Skotnicova, Dvorackova, Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein-specific T cells in lung transplant recipients, Transplantation, doi:10.1097/TP.0000000000004021
Havlin, Svorcova, Dvorackova, Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients, J Heart Lung Transplant, doi:10.1016/j.healun.2021.05.004
Heldman, Kates, Safa, Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic, Am J Transplant, doi:10.1111/ajt.16840
Hoffmann, Krüger, Schulz, The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic, Cell, doi:10.1016/j.cell.2021.12.032
Hui, Ho, Cheung, Omicron variant replication in human bronchus and lung ex vivo, Nature, doi:10.1038/s41586-022-04479-6
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature, doi:10.1038/s41586-022-04594-4
Kamp, Hinrichs, Fuge, Ewen, Gottlieb, COVID-19 in lung transplant recipients-risk prediction and outcomes, PLoS One, doi:10.1371/journal.pone.0257807
Koczulla, Sczepanski, Koteczki, SARS-CoV-2 infection in two patients following recent lung transplantation, Am J Transplant, doi:10.1111/ajt.15998
Maslo, Friedland, Toubkin, Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves, JAMA, doi:10.1001/jama.2021.24868
Pulliam, Van Schalkwyk, Govender, Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa, Science, doi:10.1126/science.abn4947
Ravanan, Mumford, Ushiro-Lumb, Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis, Transplantation, doi:10.1097/TP.0000000000003908
Salerno, Jennings, Lange, Kovac, Shertel et al., Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients, Am J Transplant, doi:10.1111/ajt.17027
Takashita, Kinoshita, Yamayoshi, Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Uraki, Kiso, Iida, Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA, Nature, doi:10.1038/s41586-022-04856-1
Verleden, Glanville, Lease, Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-a consensus report from the pulmonary council of the ISHLT, J Heart Lung Transplant, doi:10.1016/j.healun.2019.03.009
Wolter, Jassat, Walaza, Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study, Lancet, doi:10.1016/S0140-6736(22)00017-4
Wratil, Stern, Priller, Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern, Nat Med, doi:10.1038/s41591-022-01715-4
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention, JAMA, doi:10.1001/jama.2020.2648
DOI record:
{
"DOI": "10.1007/s15010-022-01914-8",
"ISSN": [
"0300-8126",
"1439-0973"
],
"URL": "http://dx.doi.org/10.1007/s15010-022-01914-8",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Purpose</jats:title>\n <jats:p>Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is currently the major threat for immunocompromised individuals. The course of COVID-19 in lung transplant recipients in the Omicron era remains unknown. The aim of the study was to assess outcome and associated factors in lung transplant recipients in a German-wide multicenter approach.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>All affected individuals from January 1st to March 20th, 2022 from 8 German centers during the Omicron wave were collected. Baseline characteristics and antiviral measures were associated with outcome.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of 218 patients with PCR-proven SARS-CoV-2 infection 166 patients (76%) received any early (< 7 days) antiviral therapy median 2 (interquartile range 1–4) days after symptom onset. Most patients received sotrovimab (57%), followed by remdesivir (21%) and molnupiravir (21%). An early combination therapy was applied in 45 patients (21%). Thirty-four patients (16%) developed a severe or critical disease severity according to the WHO scale. In total, 14 patients (6.4%) died subsequently associated with COVID-19. Neither vaccination and antibody status, nor applied treatments were associated with outcome. Only age and glomerular filtration rate < 30 ml/min/1.73m<jats:sup>2</jats:sup> were independent risk factors for a severe or critical COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>COVID-19 due to Omicron remains an important threat for lung transplant recipients. In particular, elderly patients and patients with impaired kidney function are at risk for worse outcome. Prophylaxis and therapy in highly immunocompromised individuals need further improvement.</jats:p>\n </jats:sec>",
"alternative-id": [
"1914"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "7 July 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "25 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "9 September 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interests."
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The study was approved by the central institutional ethics committee (LMU Munich, Germany; project number 22-0078)."
}
],
"author": [
{
"affiliation": [],
"family": "Kneidinger",
"given": "Nikolaus",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hecker",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bessa",
"given": "Vasiliki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hettich",
"given": "Ina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wald",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wege",
"given": "Sabine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nolde",
"given": "Anna-Barbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oldigs",
"given": "Maike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Syunyaeva",
"given": "Zulfiya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkens",
"given": "Heinrike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gottlieb",
"given": "Jens",
"sequence": "additional"
}
],
"container-title": "Infection",
"container-title-short": "Infection",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
9
]
],
"date-time": "2022-09-09T12:37:36Z",
"timestamp": 1662727056000
},
"deposited": {
"date-parts": [
[
2022,
9,
9
]
],
"date-time": "2022-09-09T12:45:17Z",
"timestamp": 1662727517000
},
"funder": [
{
"name": "Universitätsklinik München"
}
],
"indexed": {
"date-parts": [
[
2022,
9,
9
]
],
"date-time": "2022-09-09T13:14:24Z",
"timestamp": 1662729264827
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
9,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
9
]
],
"date-time": "2022-09-09T00:00:00Z",
"timestamp": 1662681600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
9
]
],
"date-time": "2022-09-09T00:00:00Z",
"timestamp": 1662681600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s15010-022-01914-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s15010-022-01914-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s15010-022-01914-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
9,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
9
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1111/ajt.16369",
"author": "E Coll",
"doi-asserted-by": "publisher",
"first-page": "1825",
"journal-title": "Am J Transplant",
"key": "1914_CR1",
"unstructured": "Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21:1825–37. https://doi.org/10.1111/ajt.16369.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1111/ajt.15998",
"author": "RA Koczulla",
"doi-asserted-by": "publisher",
"first-page": "2928",
"journal-title": "Am J Transplant",
"key": "1914_CR2",
"unstructured": "Koczulla RA, Sczepanski B, Koteczki A, et al. SARS-CoV-2 infection in two patients following recent lung transplantation. Am J Transplant. 2020;20:2928–32. https://doi.org/10.1111/ajt.15998.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1111/ajt.16241",
"author": "M Aversa",
"doi-asserted-by": "publisher",
"first-page": "3072",
"journal-title": "Am J Transplant",
"key": "1914_CR3",
"unstructured": "Aversa M, Benvenuto L, Anderson M, et al. COVID-19 in lung transplant recipients: a single center case series from New York City. Am J Transplant. 2020;20:3072–80. https://doi.org/10.1111/ajt.16241.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0257807",
"author": "JC Kamp",
"doi-asserted-by": "publisher",
"journal-title": "PLoS One",
"key": "1914_CR4",
"unstructured": "Kamp JC, Hinrichs JB, Fuge J, Ewen R, Gottlieb J. COVID-19 in lung transplant recipients—risk prediction and outcomes. PLoS One. 2021;16: e0257807. https://doi.org/10.1371/journal.pone.0257807.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16840",
"author": "MR Heldman",
"doi-asserted-by": "publisher",
"first-page": "279",
"journal-title": "Am J Transplant",
"key": "1914_CR5",
"unstructured": "Heldman MR, Kates OS, Safa K, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant. 2022;22:279–88. https://doi.org/10.1111/ajt.16840.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000003908",
"author": "R Ravanan",
"doi-asserted-by": "publisher",
"first-page": "e263",
"journal-title": "Transplantation",
"key": "1914_CR6",
"unstructured": "Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021;105:e263–4. https://doi.org/10.1097/TP.0000000000003908.",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1183/13993003.00124-2022",
"author": "J Gottlieb",
"doi-asserted-by": "publisher",
"journal-title": "Eur Respir J.",
"key": "1914_CR7",
"unstructured": "Gottlieb J, Kolditz M, Gade N, Welte T, Kneidinger N. Benefit of monoclonal antibodies in early treatment of COVID-19 after lung transplantation—a retrospective analysis in two centers. Eur Respir J. 2022. https://doi.org/10.1183/13993003.00124-2022.",
"year": "2022"
},
{
"DOI": "10.1126/science.abn4947",
"author": "JRC Pulliam",
"doi-asserted-by": "publisher",
"journal-title": "Science.",
"key": "1914_CR8",
"unstructured": "Pulliam JRC, van Schalkwyk C, Govender N, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022. https://doi.org/10.1126/science.abn4947.",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2021.12.032",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "Cell.",
"key": "1914_CR9",
"unstructured": "Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447-456.e11. https://doi.org/10.1016/j.cell.2021.12.032.",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04479-6",
"author": "KPY Hui",
"doi-asserted-by": "publisher",
"first-page": "715",
"journal-title": "Nature.",
"key": "1914_CR10",
"unstructured": "Hui KPY, Ho JCW, Cheung MC, et al. Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–20. https://doi.org/10.1038/s41586-022-04479-6.",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00017-4",
"author": "N Wolter",
"doi-asserted-by": "publisher",
"first-page": "437",
"journal-title": "Lancet.",
"key": "1914_CR11",
"unstructured": "Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–46. https://doi.org/10.1016/S0140-6736(22)00017-4.",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1101/2022.01.26.22269927",
"doi-asserted-by": "publisher",
"key": "1914_CR12",
"unstructured": "Fall A, Eldesouki RE, Sachithanandham J, et al. A quick displacement of the SARS-CoV-2 variant delta with omicron: unprecedented spike in COVID-19 cases associated with fewer admissions and comparable upper respiratory viral loads. medRxiv. 2022. https://doi.org/10.1101/2022.01.26.22269927"
},
{
"DOI": "10.1056/NEJMoa2107934",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"journal-title": "N Engl J Med.",
"key": "1914_CR13",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–50. https://doi.org/10.1056/NEJMoa2107934.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1236",
"journal-title": "JAMA.",
"key": "1914_CR14",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–46. https://doi.org/10.1001/jama.2022.2832.",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "1914_CR15",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. https://doi.org/10.1056/NEJMoa2116044"
},
{
"DOI": "10.1038/s41591-022-01715-4",
"author": "PR Wratil",
"doi-asserted-by": "publisher",
"first-page": "496",
"issue": "3",
"journal-title": "Nat Med.",
"key": "1914_CR16",
"unstructured": "Wratil PR, Stern M, Priller A, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28(3):496–503. https://doi.org/10.1038/s41591-022-01715-4.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/j.healun.2019.03.009",
"author": "GM Verleden",
"doi-asserted-by": "publisher",
"first-page": "493",
"journal-title": "J Heart Lung Transplant.",
"key": "1914_CR17",
"unstructured": "Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment—a consensus report from the pulmonary council of the ISHLT. J Heart Lung Transplant. 2019;38:493–503. https://doi.org/10.1016/j.healun.2019.03.009.",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA.",
"key": "1914_CR18",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–42. https://doi.org/10.1001/jama.2020.2648.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.7326/M22-0308",
"author": "D Bouzid",
"doi-asserted-by": "publisher",
"journal-title": "Ann Intern Med.",
"key": "1914_CR19",
"unstructured": "Bouzid D, Visseaux B, Kassasseya C, et al. Comparison of patients infected with delta versus omicron COVID-19 variants presenting to paris emergency departments: a retrospective cohort study. Ann Intern Med. 2022. https://doi.org/10.7326/M22-0308.",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.24868",
"author": "C Maslo",
"doi-asserted-by": "publisher",
"first-page": "583",
"journal-title": "JAMA.",
"key": "1914_CR20",
"unstructured": "Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327:583–4. https://doi.org/10.1001/jama.2021.24868.",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004021",
"author": "J Havlin",
"doi-asserted-by": "publisher",
"first-page": "e183",
"journal-title": "Transplantation.",
"key": "1914_CR21",
"unstructured": "Havlin J, Skotnicova A, Dvorackova E, et al. Impaired humoral response to third dose of BNT162b2 mRNA COVID-19 vaccine despite detectable spike protein-specific T cells in lung transplant recipients. Transplantation. 2022;106:e183–4. https://doi.org/10.1097/TP.0000000000004021.",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1016/j.healun.2021.05.004",
"author": "J Havlin",
"doi-asserted-by": "publisher",
"first-page": "754",
"journal-title": "J Heart Lung Transplant.",
"key": "1914_CR22",
"unstructured": "Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–8. https://doi.org/10.1016/j.healun.2021.05.004.",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N Engl J Med.",
"key": "1914_CR23",
"unstructured": "Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386:305–15. https://doi.org/10.1056/NEJMoa2116846.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17027",
"author": "DM Salerno",
"doi-asserted-by": "publisher",
"journal-title": "Am J Transplant.",
"key": "1914_CR24",
"unstructured": "Salerno DM, Jennings DL, Lange NW, Kovac DB, Shertel T, Chen JK, Hedvat J, Scheffert J, Brown RS Jr, Pereira MR. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022. https://doi.org/10.1111/ajt.17027.",
"year": "2022"
},
{
"DOI": "10.1101/2022.05.03.22274524",
"doi-asserted-by": "publisher",
"key": "1914_CR25",
"unstructured": "Gleeson S, Martin P, Thomson T, et al. Kidney transplant recipients and omicron: outcomes, effect of vaccines and the efficacy and safety of novel treatments. medRxiv. https://doi.org/10.1101/2022.05.03.22274524"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"author": "S Iketani",
"doi-asserted-by": "publisher",
"first-page": "553",
"journal-title": "Nature.",
"key": "1914_CR26",
"unstructured": "Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–6. https://doi.org/10.1038/s41586-022-04594-4.",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "publisher",
"key": "1914_CR27",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. https://doi.org/10.1056/NEJMc2201933"
},
{
"DOI": "10.1038/s41586-022-04856-1",
"doi-asserted-by": "publisher",
"key": "1914_CR28",
"unstructured": "Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron/BA.2. Nature. 2022. https://doi.org/10.1038/s41586-022-04856-1"
},
{
"DOI": "10.1097/TP.0000000000003670",
"author": "YB Hadi",
"doi-asserted-by": "publisher",
"first-page": "1365",
"journal-title": "Transplantation.",
"key": "1914_CR29",
"unstructured": "Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105:1365–71. https://doi.org/10.1097/TP.0000000000003670.",
"volume": "105",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s15010-022-01914-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a Nationwide German study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}
kneidinger
