Antimalarials are not Effective as Pre-Exposure Prophylaxis for COVID-19: A Retrospective Matched Control Study
et al., Journal of Drugs in Dermatology, doi:10.36849/jdd.6593, Jul 2023
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
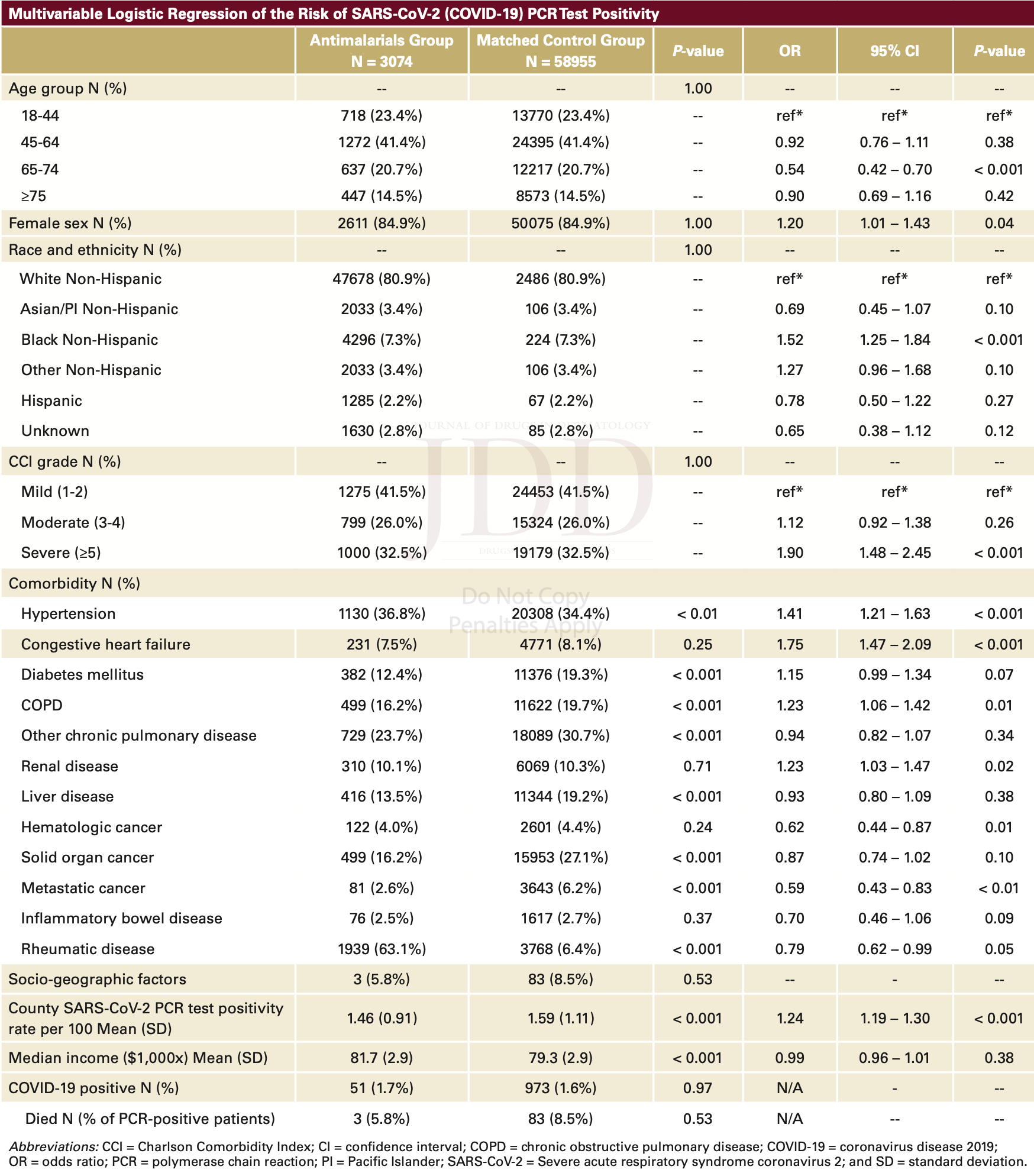
Retrospective 3,074 patients with antimalarial prescriptions and 58,955 matched controls, showing no significant differences with antimalarial prophylaxis for PCR+ cases (99% HCQ). Authors provide only PCR+ and mortality outcomes, and do not provide intermediate clinical outcomes that may show a statistically significant benefit. Authors do not adjust for the very different baseline risk for systemic autoimmune disease patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.0011 (for symptomatic disease).
Although the 31% lower mortality is not statistically significant, it is consistent with the significant 27% lower mortality [22‑31%] from meta-analysis of the 256 mortality results to date.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 30.6% lower, RR 0.69, p = 0.80, treatment 3 of 3,074 (0.1%), control 83 of 58,995 (0.1%), NNT 2320.
|
|
risk of case, 5.9% higher, RR 1.06, p = 0.70, treatment 51 of 3,074 (1.7%), control 973 of 58,995 (1.6%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Klebanov et al., 1 Jul 2023, retrospective, USA, peer-reviewed, 10 authors.
Contact: sales@jddonline.com, support@jddonline.com.
Antimalarials are not Effective as Pre-Exposure Prophylaxis for COVID-19: A Retrospective Matched Control Study
Journal of Drugs in Dermatology, doi:10.36849/jdd.6593
exposure impact on SARS-CoV-2 risk is of great importance to the practicing dermatologist. We investigated the efficacy of antimalarial drugs as pre-exposure SARS-CoV-2 prophylaxis in a US tertiary-care center.
MATERIALS AND METHODS We included all adult patients with at least one prescription for chloroquine, hydroxychloroquine, or quinacrine from July 1, 2019 to February 29, 2020 (limiting prescriptions to those started before the pandemic onset) in the MassGeneral Brigham Enterprise Data Warehouse and Research Patient Data Registry. We exactmatched antimalarial-treated study patients with controls on age, sex, race, and Charleston Comorbidity Index. Additional collected variables included zip codes (used to estimate income using 2010 US Census), and medical history using ICD-9/ICD-10
References
Boulware, Pullen, Bangdiwala, Pastick, Lofgren et al., A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19, N Engl J Med
Carafoli, Chloroquine and hydroxychloroquine in the prophylaxis and therapy of COVID-19 infection
Favalli, Agape, Caporali, Incidence and clinical course of COVID-19 in patients with connective tissue diseases: A descriptive observational analysis, J Rheumatol
Hooijberg, Boekel, Vogelzang, Leeuw, Boers et al., Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population, Lancet Rheumatol
Kuderer, Choueiri, Shah, Shyr, Rubinstein et al., Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study, Lancet
Robinson, Gyawali, Evans, COVID-19 and cancer: do we really know what we think we know?, Nat Rev Clin Oncol
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review, JAMA -J Am Med Assoc
Schreiber, Sciascia, Bruce, Giles, Cuadrado et al., Hydroxychloroquine in patients with rheumatic diseases during the COVID-19 pandemic: a letter to clinicians, Lancet Rheumatol
DOI record:
{
"DOI": "10.36849/jdd.6593",
"ISSN": [
"1545-9616"
],
"URL": "http://dx.doi.org/10.36849/jdd.6593",
"author": [
{
"affiliation": [],
"family": "Klebanov",
"given": "Nikokai",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pahalyants",
"given": "Vartan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Said",
"given": "Jordan T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murphy",
"given": "William. S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Theodosakis",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scarry",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duey",
"given": "Stacey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klevens",
"given": "Monina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lilly",
"given": "Evelyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Semenov",
"given": "Yevgeniy R.",
"sequence": "additional"
}
],
"container-title": "Journal of Drugs in Dermatology",
"container-title-short": "JDD",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
9
]
],
"date-time": "2023-08-09T17:55:51Z",
"timestamp": 1691603751000
},
"deposited": {
"date-parts": [
[
2023,
8,
9
]
],
"date-time": "2023-08-09T17:56:17Z",
"timestamp": 1691603777000
},
"indexed": {
"date-parts": [
[
2023,
8,
10
]
],
"date-time": "2023-08-10T04:31:28Z",
"timestamp": 1691641888059
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
7,
1
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
7,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
8,
1
]
]
}
},
"member": "22713",
"original-title": [],
"page": "840-843",
"prefix": "10.36849",
"published": {
"date-parts": [
[
2023,
7,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
8,
1
]
]
},
"publisher": "SanovaWorks",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://jddonline.com/articles/dermatology/S1545961623P0840X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Antimalarials are not Effective as Pre-Exposure Prophylaxis for COVID-19: A Retrospective Matched Control Study",
"type": "journal-article",
"volume": "22"
}
