Potential drug interactions with nirmatrelvir/ritonavir in critically ill patients with COVID-19 – a retrospective observational study
et al., RPS Pharmacy and Pharmacology Reports, doi:10.1093/rpsppr/rqae028, Feb 2025
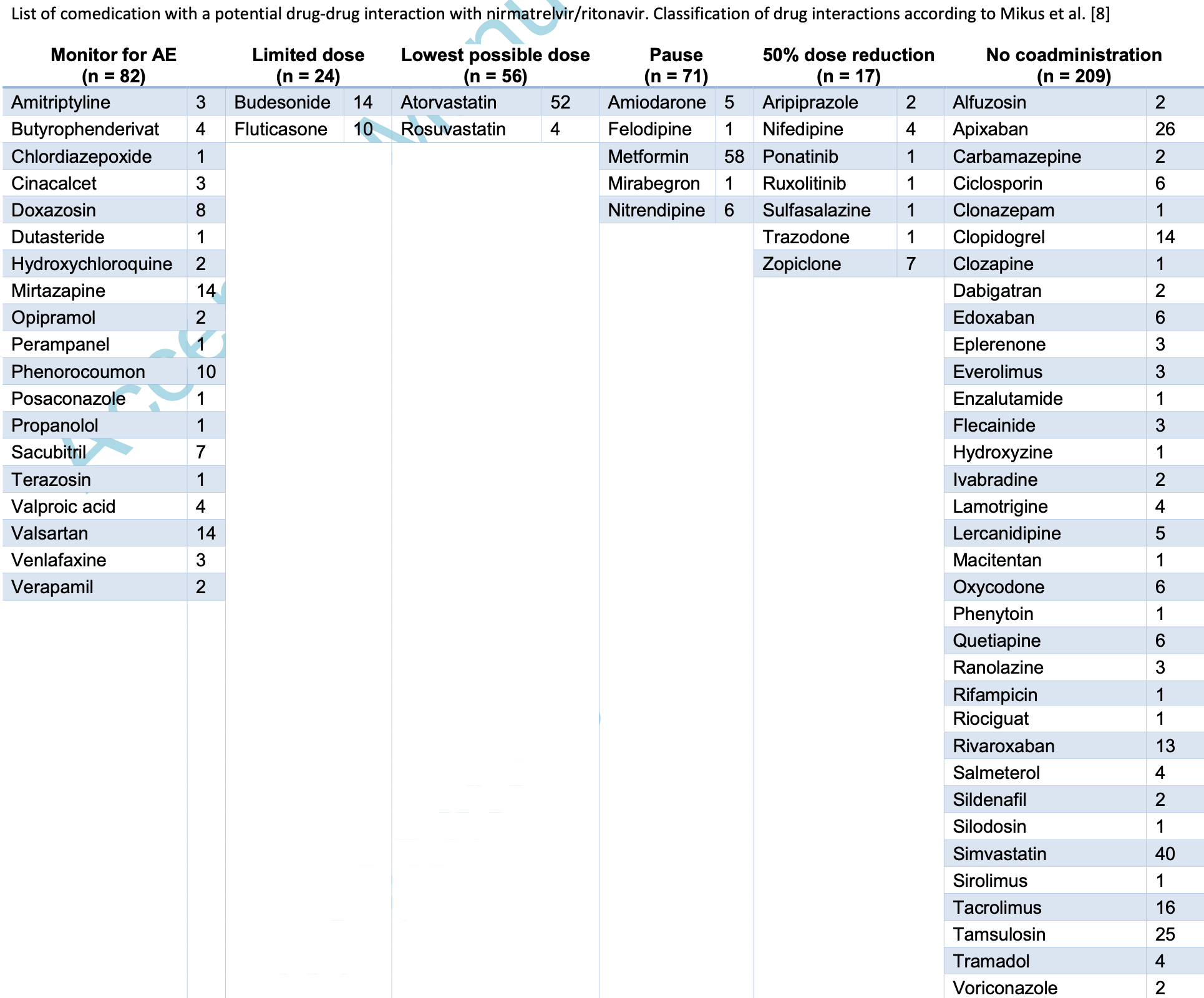
Retrospective 500 critically ill COVID-19 patients in Germany showing potential drug-drug interactions with paxlovid in 48% of patients, with higher age and number of comorbidities significantly associated with drug-drug interactions. Authors note that the population that most needs effective treatment also has the greatest risk of drug-drug interactions.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Jarczak et al., 15 Feb 2025, retrospective, Germany, peer-reviewed, 9 authors, study period March 2020 - June 2022.
Contact: mar.fischer@uke.de.
Potential drug interactions with nirmatrelvir/ritonavir in critically ill patients with COVID-19 -a retrospective observational study
doi:10.1093/rpsppr/rqae028/8016069
Objectives Nirmatrelvir/ritonavir is recommended for high-risk patients with COVID-19 to reduce disease progression and mortality. Ritonavir significantly increases the bioavailability of nirmatrelvir and is the most potent irreversible cytochrome P 450 3A4 inhibitor in clinical use, resulting in a substantial risk for drug-drug interactions (DDI). We aimed to analyze the incidence of potential DDI (pDDI) in critically ill patients with SARS-CoV-2 infection.
Methods This is a retrospective single-center study in a quaternary care center in Northern Germany. We reviewed electronic health records for demographic characteristics, comorbid conditions, and medication history. The pre-existing comedication was screened for pDDI with nirmatrelvir/ritonavir using publicly available databases. Binary logistic regression was used to identify patient characteristics associated with pDDI. 2.
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Hu, Xiong, Zhu, Zhang, Zhang et al., The SARS-CoV-2 main protease (Mpro): Structure, function, and emerging therapies for COVID-19, MedComm
Loos, Beijnen, Schinkel, The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism?, Int J Mol Sci
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Prescribing Nirmatrelvir-Ritonavir: How to Recognize and Manage Drug-Drug Interactions, Ann Intern Med
Mikus, Foerster, Terstegen, Vogt, Said et al., Oral drugs against COVID-19, Dtsch Ärzteblatt Int
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin Infect Dis
Ng, Correia, Seagal, Degoey, Schrimpf et al., Antiviral Drug Discovery for the Treatment of COVID-19 Infections, Viruses
Organization, WHO recommends highly successful COVID-19 therapy and calls for wide geographical distribution and transparency from originator
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19, Science
Trauth, Respiratorische Virusinfektionen, Med Klin -Intensiv Notfallmedizin
Tregoning, Flight, Higham, Wang, Pierce, Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape, Nat Rev Immunol
Xiao, Mehta, Curran, Garibaldi, Alexander, Consortium NCCC (N3C). Potential drug-drug interactions among U.S. adults treated with nirmatrelvir/ritonavir: A cross-sectional
DOI record:
{
"DOI": "10.1093/rpsppr/rqae028",
"ISSN": [
"2754-5849"
],
"URL": "http://dx.doi.org/10.1093/rpsppr/rqae028",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Objectives</jats:title>\n <jats:p>Nirmatrelvir/ritonavir is recommended for high-risk patients with COVID-19 to reduce disease progression and mortality. Ritonavir significantly increases the bioavailability of nirmatrelvir and is the most potent irreversible cytochrome P 450 3A4 inhibitor in clinical use, resulting in a substantial risk for drug-drug interactions (DDI). We aimed to analyze the incidence of potential DDI (pDDI) in critically ill patients with SARS-CoV-2 infection.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This is a retrospective single-center study in a quaternary care center in Northern Germany. We reviewed electronic health records for demographic characteristics, comorbid conditions, and medication history. The pre-existing comedication was screened for pDDI with nirmatrelvir/ritonavir using publicly available databases. Binary logistic regression was used to identify patient characteristics associated with pDDI.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Key Findings</jats:title>\n <jats:p>Of 500 critically ill patients with SARS-CoV-2 infection, 362 (72.4%) received pre-existing comedication. A total of 241/500 patients (48.2%) had a medication history prone to pDDI. Antidiabetics, lipid-lowering drugs, and anticoagulants were among the most frequently used agents with a pDDI. Higher age (OR 1.043; 1.028-1.058; p&lt;0.01) and the number of comorbidities (OR 1.229; 1.119-1.350; p&lt;0.01) were significantly associated with pDDI.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The very patient population that may benefit most from treatment with nirmatrelvir/ritonavir also has the greatest risk of pDDI. Polypharmacy is frequently present in these patients and a conscientious check of the comedication is mandatory before a treatment with nirmatrelvir/ritonavir can be initiated.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"family": "Jarczak",
"given": "Dominik",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"family": "König",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, General Hospital of Heidenheim , 89522 Heidenheim,",
"place": [
"Germany"
]
}
],
"family": "Röhr",
"given": "Anka C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"family": "Forstreuter",
"given": "Anika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, I. Department of Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
},
{
"name": "German Center for Infection Research (DZIF) , Partner Site Hamburg-Lübeck-Borstel-Riems,",
"place": [
"Germany"
]
}
],
"family": "Brehm",
"given": "Thomas Theo",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5033-1938",
"affiliation": [
{
"name": "Division of Infectious Diseases, I. Department of Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"authenticated-orcid": false,
"family": "Schulze zur Wiesch",
"given": "Julian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"family": "Roedl",
"given": "Kevin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8391-3988",
"affiliation": [
{
"name": "Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"authenticated-orcid": false,
"family": "Kluge",
"given": "Stefan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7530-8155",
"affiliation": [
{
"name": "Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf , Hamburg,",
"place": [
"Germany"
]
}
],
"authenticated-orcid": false,
"family": "Fischer",
"given": "Marlene",
"sequence": "additional"
}
],
"container-title": "RPS Pharmacy and Pharmacology Reports",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
2,
15
]
],
"date-time": "2025-02-15T13:27:16Z",
"timestamp": 1739626036000
},
"deposited": {
"date-parts": [
[
2025,
2,
15
]
],
"date-time": "2025-02-15T13:27:16Z",
"timestamp": 1739626036000
},
"indexed": {
"date-parts": [
[
2025,
2,
16
]
],
"date-time": "2025-02-16T05:06:57Z",
"timestamp": 1739682417453,
"version": "3.37.1"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
2,
15
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
15
]
],
"date-time": "2025-02-15T00:00:00Z",
"timestamp": 1739577600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/rpsppr/advance-article-pdf/doi/10.1093/rpsppr/rqae028/61902989/rqae028.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/rpsppr/advance-article-pdf/doi/10.1093/rpsppr/rqae028/61902989/rqae028.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
2,
15
]
]
},
"published-online": {
"date-parts": [
[
2025,
2,
15
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/rpsppr/advance-article/doi/10.1093/rpsppr/rqae028/8016069"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Potential drug interactions with nirmatrelvir/ritonavir in critically ill patients with COVID-19 – a retrospective observational study",
"type": "journal-article"
}