Clinical and molecular landscape of prolonged SARS-CoV-2 infection with resistance to remdesivir in immunocompromised patients
et al., PNAS Nexus, doi:10.1093/pnasnexus/pgaf085, Mar 2025
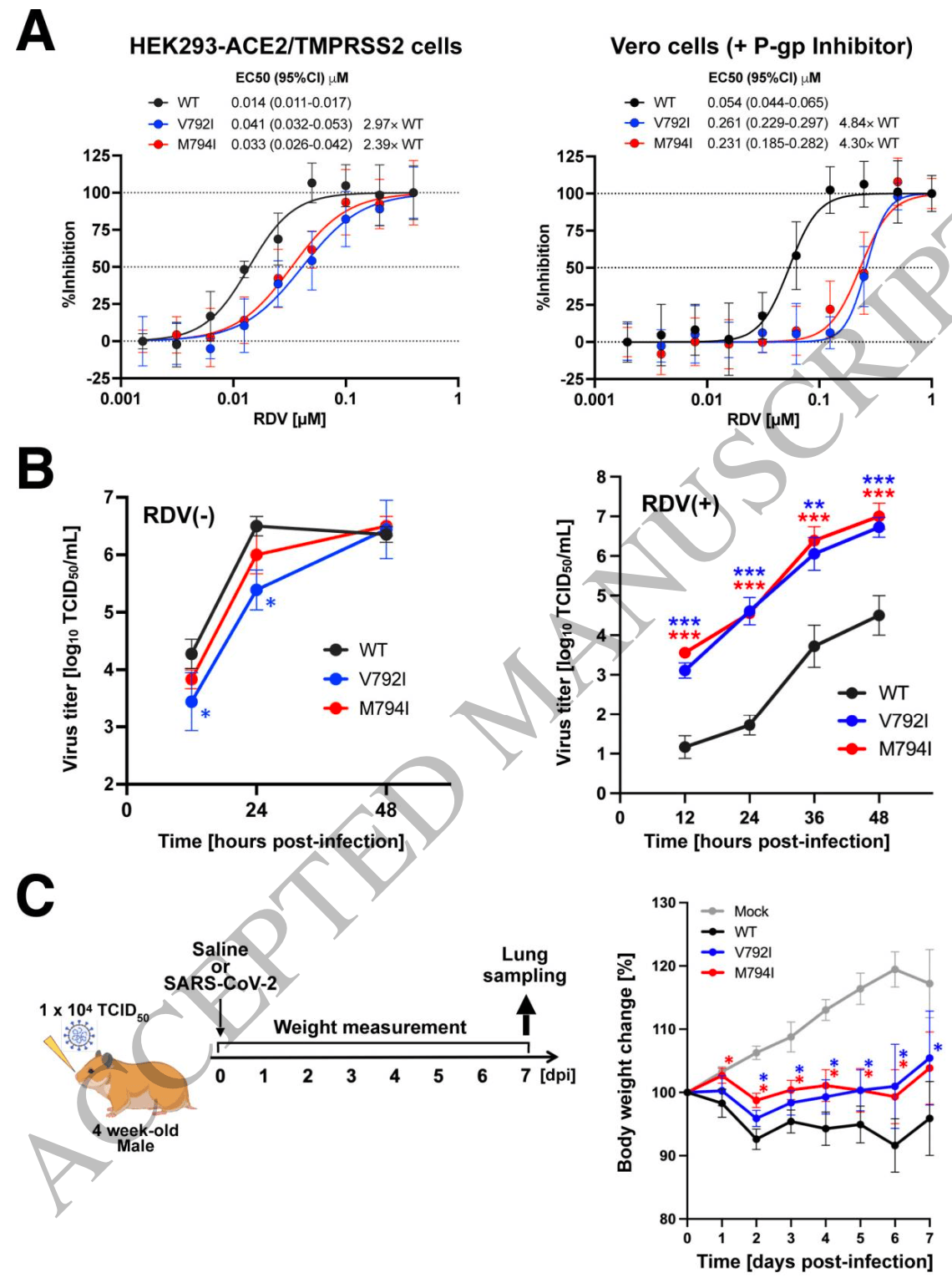
Clinical and virological study of 3 immunocompromised B-cell lymphoma patients with prolonged SARS-CoV-2 infection showing development of remdesivir and sotrovimab resistance. Through serial viral genome sequencing, authors identified NSP12 mutations that conferred 2.4-4.8-fold reduced susceptibility to remdesivir. Using recombinant viruses, they demonstrated that these mutations conveyed remdesivir resistance while attenuating viral pathogenicity in hamsters. The study also found resistance to sotrovimab in one patient through spike protein mutations (E340A, E340D, F342INS).
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Study covers remdesivir and sotrovimab.
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Iriyama et al., 18 Mar 2025, Japan, peer-reviewed, 30 authors.
Contact: atomita@fujita-hu.ac.jp, fukut@pop.med.hokudai.ac.jp.
Clinical and molecular landscape of prolonged SARS-CoV-2 infection with resistance to remdesivir in immunocompromised patients
doi:10.1093/pnasnexus/pgaf085/8083008
Patients with hematologic diseases have experienced COVID-19 with prolonged, progressive course. Here we present clinical, pathological, and virological analyses of three cases of prolonged COVID-19 among patients undergoing treatment for B-cell lymphoma. These patients had all been treated with anti-CD20 antibody and bendamustine. Despite various antiviral treatments, high SARS-CoV-2 levels persisted for more than 4 weeks, and two of them succumbed to COVID-19. Autopsy showed bronchopneumonia, interstitial pneumonia, alveolar hemorrhage, and fibrosis. Overlapping CMV, fungal and/or bacterial infections were also confirmed. Sequencing of SARS-CoV-2 showed accumulation of mutations and changes in
Significance statement In immunocompromised patients with hematological malignancies, prolonged/progressive course of COVID-19 is still a critical problem, even in an era when vaccines and several antiviral therapeutics have been developed. Here we analyzed serial viral specimens of three B-cell lymphoma patients undergoing immuno-chemotherapies who experienced prolonged/progressive course of COVID-19 and demonstrated that diverse genetic mutations on SARS-CoV-2 accumulated on therapy within each individual. In vitro drug susceptibility assay revealed that specific genetic alterations on NSP12 (V792I and M794I) were responsible for acquisition of remdesivir resistance. These findings indicate that mutations related to drug resistance accumulate in SARS-CoV-2 in immunocompromised patients, suggesting the importance of treatment strategies that can eradicate the virus at an early stage of COVID-19 onset. (116 words)
Authorship contribution
Sequence data availability The RNAseq data of case #1 specimens 1 to 12 in Figure 1A will be available from NCBI SRA (PRJNA1049969). The data of case #1 specimen 13 in Figure 1A and Supplementary Figure S2 (at autopsy) will be available from DDBJ DRA (DRA017735). The sequence data of case #2 and 3 are available from DDBJ (accession numbers: case #2. LC798944, LC753061, LC753062; case #3. LC798945, LC798946, LC798947).
References
Atanackovic, Kreitman, Cohen, T cell responses against SARS-CoV-2 and its Omicron variant in a patient with B cell lymphoma after multiple doses of a COVID-19 mRNA vaccine, J Immunother Cancer
Awadasseid, Wu, Tanaka, Zhang, Effective drugs used to combat SARS-CoV-2 infection and the current status of vaccines, Biomed Pharmacother
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Dewolf, Laracy, Perales, Kamboj, Van Den Brink et al., SARS-CoV-2 in immunocompromised individuals, Immunity
Dulery, Lamure, Delord, Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy, Am J Hematol
Flaifel, Kwok, Ko, Pulmonary Pathology of End-Stage COVID-19 Disease in Explanted Lungs and Outcomes After Lung Transplantation, Am J Clin Pathol
Focosi, Maggi, Mcconnell, Casadevall, Very low levels of remdesivir resistance in SARS-COV-2 genomes after 18 months of massive usage during the COVID19 pandemic: A GISAID exploratory analysis, Antiviral Res
Franceschini, Pellegrino, Todisco, Persistent SARS-CoV-2 infection with multiple clinical relapses in two patients with follicular lymphoma treated with bendamustine and obinutuzumab or rituximab, Infection
Gandhi, Klein, Robertson, De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report, Nat Commun
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Hiraoka, Kikuchi, Yamauchi, Purine analog-like properties of bendamustine underlie rapid activation of DNA damage response and synergistic effects with pyrimidine analogues in lymphoid malignancies, PLoS One
Hogan, Doohan, Wu, Estimating long-term vaccine effectiveness against SARS-CoV-2 variants: a model-based approach, Nat Commun
Huygens, Munnink, Gharbharan, Koopmans, Rijnders, Sotrovimab Resistance and Viral Persistence After Treatment of Immunocompromised Patients Infected With the Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant, Clin Infect Dis
Ichikawa, Tamura, Takahata, Prolonged shedding of viable SARS-CoV-2 in immunocompromised patients with haematological malignancies: A prospective study, Br J Haematol
Ikeda, Fukumoto, Uesugi, Clinical and immunological characteristics of prolonged SARS-CoV-2 Omicron infection in hematologic disease, Blood Cancer J
Imai, Ito, Kiso, Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB, N Engl J Med
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Kabinger, Stiller, Schmitzova, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol
Krivosikova, Kuracinova, Martanovic, Long COVID Complicated by Fatal Cytomegalovirus and Aspergillus Infection of the Lungs: An Autopsy Case Report, Viruses
Levin, Ustianowski, Wit, Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, N Engl J Med
Li, Wu, Wang, Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China, Histopathology
Mangieri, Valenzuela, Solsky, Switching Perfusion Agents for Repeat Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy: Surgical Dogma or Evidence-Based Practice?, Ann Surg Oncol
Martinez-Calle, Hartley, Ahearne, Kinetics of T-cell subset reconstitution following treatment with bendamustine and rituximab for low-grade lymphoproliferative disease: a population-based analysis, Br J Haematol
Matsuyama, Nao, Shirato, Enhanced isolation of SARS-CoV-2 by TMPRSS2expressing cells, Proc Natl Acad Sci U S A
Miller, Hachmann, Collier, Substantial Neutralization Escape by SARS-CoV-2 Omicron Variants BQ.1.1 and XBB
Motozono, Toyoda, Zahradnik, SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity, Cell Host Microbe
Nooruzzaman, Johnson, Emergence of transmissible SARS-CoV-2 variants with decreased sensitivity to antivirals in immunocompromised patients with persistent infections, Nat Commun
Okamoto, Fujigaki, Iriyama, CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma, Blood Adv
Orth, Flasshove, Berger, Early combination therapy of COVID-19 in high-risk patients, Infection
Pagano, Salmanton-Garcia, Marchesi, Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: results from the EPICOVIDEHA survey, Blood
Perry, Luttwak, Balaban, Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma, Blood Adv
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med
Rockett, Basile, Maddocks, Resistance Mutations in SARS-CoV-2 Delta Variant after Sotrovimab Use, N Engl J Med
Rosa, Sillence, Ackerley, Lingwood, Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis, J Biol Chem
Rueda, Santos, Angarita, Can presence of HLA type I and II alleles be associated with clinical spectrum of CHIKV infection?, Transbound Emerg Dis
Stevens, Pruijssers, Lee, Mutations in the SARS-CoV-2 RNA-dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms, Sci Transl Med
Takashita, Kinoshita, Yamayoshi, Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant, N Engl J Med
Tomiyama, Suzuki, Harada, A third dose of the BNT162b2 mRNA vaccine sufficiently improves the neutralizing activity against SARS-CoV-2 variants in liver transplant recipients, Front Cell Infect Microbiol
Torii, Kim, Koseki, Increased flexibility of the SARS-CoV-2 RNA-binding site causes resistance to remdesivir, PLoS Pathog
Torii, Ono, Suzuki, Establishment of a reverse genetics system for SARS-CoV-2 using circular polymerase extension reaction, Cell Rep
Tsutsumi, Ito, Horikita, Moriki, Teshima, COVID-19 antibody production by vaccination in chemotherapy with CD20 antibody for B-cell lymphoma, Mol Clin Oncol
Tuekprakhon, Nutalai, Dijokaite-Guraliuc, Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum, Cell
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Visco, Marcheselli, Mina, A prognostic model for patients with lymphoma and COVID-19: a multicentre cohort study, Blood Adv
Wang, Guo, Iketani, Antibody evasion by SARS-CoV-2 Omicron subvariants BA, BA.4 and BA.5
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Zsichla, Muller, Risk Factors of Severe COVID-19: A Review of Host, Viral and Environmental Factors, Viruses
DOI record:
{
"DOI": "10.1093/pnasnexus/pgaf085",
"ISSN": [
"2752-6542"
],
"URL": "http://dx.doi.org/10.1093/pnasnexus/pgaf085",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Patients with hematologic diseases have experienced COVID-19 with prolonged, progressive course. Here we present clinical, pathological, and virological analyses of three cases of prolonged COVID-19 among patients undergoing treatment for B-cell lymphoma. These patients had all been treated with anti-CD20 antibody and bendamustine. Despite various antiviral treatments, high SARS-CoV-2 levels persisted for more than 4 weeks, and two of them succumbed to COVID-19. Autopsy showed bronchopneumonia, interstitial pneumonia, alveolar hemorrhage, and fibrosis. Overlapping CMV, fungal and/or bacterial infections were also confirmed. Sequencing of SARS-CoV-2 showed accumulation of mutations and changes in variant allele frequencies over time. NSP12 mutations V792I and M794I appeared independently in two cases as COVID-19 progressed. In vitro drug susceptibility analysis and animal experiment using recombinant SARS-CoV-2 demonstrated that each mutation, V792 and M794I, was independently responsible for remdesivir resistance and attenuated pathogenicity. E340A, E340D and F342INS mutations in the spike protein were found in one case, which may account for the sotrovimab resistance. Analysis of autopsy specimens indicated heterogeneous distribution of these mutations. In summary, we demonstrated temporal and spatial diversity in SARS-CoV-2 that evolved resistance to various antiviral agents in malignant lymphoma patients under immunodeficient conditions caused by certain types of immunochemotherapies. Strategies may be necessary to prevent acquisition of drug resistance and improve outcome, such as selection of appropriate treatment strategies for lymphoma considering patients’ immune status and institution of early intensive antiviral therapy.</jats:p>",
"author": [
{
"ORCID": "https://orcid.org/0000-0002-1709-7588",
"affiliation": [
{
"name": "Department of Hematology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Iriyama",
"given": "Chisako",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0009-0009-2765-2520",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Department of Hematology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Department of Hematology, Sapporo City General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Ichikawa",
"given": "Takaya",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1395-6610",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Institute for Vaccine Research and Development, HU-IVReD, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "One Health Research Center, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Tamura",
"given": "Tomokazu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology, Sapporo-Kosei General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Takahata",
"given": "Mutsumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology, Sapporo-Kosei General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Ishio",
"given": "Takashi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology, Sapporo-Kosei General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Ibata",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency and General Internal Medicine, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"family": "Kawai",
"given": "Ryuji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency and General Internal Medicine, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"family": "Iwata",
"given": "Mitsunaga",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4550-3499",
"affiliation": [
{
"name": "Department of Microbiology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Suzuki",
"given": "Masahiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Medical Zoology, Aichi Prefectural Institute of Public Health , Nagoya ,",
"place": [
"Japan"
]
}
],
"family": "Adachi",
"given": "Hirokazu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "One Health Research Center, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Division of International Research Promotion, International Institute for Zoonosis Control, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Nao",
"given": "Naganori",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Digzyme Inc. , Tokyo ,",
"place": [
"Japan"
]
}
],
"family": "Suzuki",
"given": "Hikoyu",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5695-8814",
"affiliation": [
{
"name": "Department of Microbiology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Kawai",
"given": "Akito",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Kamiyama",
"given": "Akifumi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3820-9542",
"affiliation": [
{
"name": "Department of Pathology, National Institute of Infectious Diseases , Tokyo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Suzuki",
"given": "Tadaki",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0009-2503-1077",
"affiliation": [
{
"name": "Department of Pathology, National Institute of Infectious Diseases , Tokyo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Hirata",
"given": "Yuichiro",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2258-9031",
"affiliation": [
{
"name": "Department of Pathology, National Institute of Infectious Diseases , Tokyo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Iida",
"given": "Shun",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5332-4434",
"affiliation": [
{
"name": "Department of Pathology, National Institute of Infectious Diseases , Tokyo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Katano",
"given": "Harutaka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, Sapporo City General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Ishii",
"given": "Yasushi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9810-7305",
"affiliation": [
{
"name": "Department of Pathology, Sapporo City General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Tsuji",
"given": "Takahiro",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3770-9684",
"affiliation": [
{
"name": "Department of Cancer Pathology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Oda",
"given": "Yoshitaka",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6470-3301",
"affiliation": [
{
"name": "Department of Cancer Pathology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Tanaka",
"given": "Shinya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, Sapporo City General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Okazaki",
"given": "Nanase",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0003-1088-5607",
"affiliation": [
{
"name": "Department of Pathology, Sapporo City General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Katayama",
"given": "Yuko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, Sapporo City General Hospital , Sapporo ,",
"place": [
"Japan"
]
}
],
"family": "Nakagawa",
"given": "Shimpei",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7502-8724",
"affiliation": [
{
"name": "Department of Diagnostic Pathology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Tsukamoto",
"given": "Tetsuya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
},
{
"name": "Center for Infectious Disease Research, Fujita Health University , Toyoake ,",
"place": [
"Japan"
]
},
{
"name": "Department of Infectious Diseases, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
},
{
"name": "Division of Infectious Diseases, University of Pittsburgh School of Medicine , Pittsburgh, PA ,",
"place": [
"USA"
]
}
],
"family": "Doi",
"given": "Yohei",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5471-8331",
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Faculty of Medicine, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Institute for Vaccine Research and Development, HU-IVReD, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "One Health Research Center, Hokkaido University , Sapporo ,",
"place": [
"Japan"
]
},
{
"name": "Laboratory of Virus Control, Research Institute for Microbial Diseases, Osaka University , Suita ,",
"place": [
"Japan"
]
},
{
"name": "AMED-CREST, Japan Agency for Medical Research and Development (AMED) , Tokyo ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Fukuhara",
"given": "Takasuke",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7228-0839",
"affiliation": [
{
"name": "Center for Infectious Disease Research, Fujita Health University , Toyoake ,",
"place": [
"Japan"
]
},
{
"name": "Department of Virology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Murata",
"given": "Takayuki",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5391-1399",
"affiliation": [
{
"name": "Department of Hematology, Fujita Health University School of Medicine , Toyoake ,",
"place": [
"Japan"
]
}
],
"authenticated-orcid": false,
"family": "Tomita",
"given": "Akihiro",
"sequence": "additional"
}
],
"container-title": "PNAS Nexus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
18
]
],
"date-time": "2025-03-18T07:44:05Z",
"timestamp": 1742283845000
},
"deposited": {
"date-parts": [
[
2025,
3,
18
]
],
"date-time": "2025-03-18T07:44:05Z",
"timestamp": 1742283845000
},
"indexed": {
"date-parts": [
[
2025,
3,
19
]
],
"date-time": "2025-03-19T04:16:40Z",
"timestamp": 1742357800933,
"version": "3.40.1"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
3,
18
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
18
]
],
"date-time": "2025-03-18T00:00:00Z",
"timestamp": 1742256000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/pnasnexus/advance-article-pdf/doi/10.1093/pnasnexus/pgaf085/62435536/pgaf085.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/pnasnexus/advance-article-pdf/doi/10.1093/pnasnexus/pgaf085/62435536/pgaf085.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
3,
18
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
18
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/pnasnexus/advance-article/doi/10.1093/pnasnexus/pgaf085/8083008"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical and molecular landscape of prolonged SARS-CoV-2 infection with resistance to remdesivir in immunocompromised patients",
"type": "journal-article"
}