Casirivimab and Imdevimab Treatment Reduces Viral Load and Improves Clinical Outcomes in Seropositive Hospitalized COVID-19 Patients with Nonneutralizing or Borderline Neutralizing Antibodies
et al., mBio, doi:10.1128/mbio.01699-22, NCT04426695, Dec 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
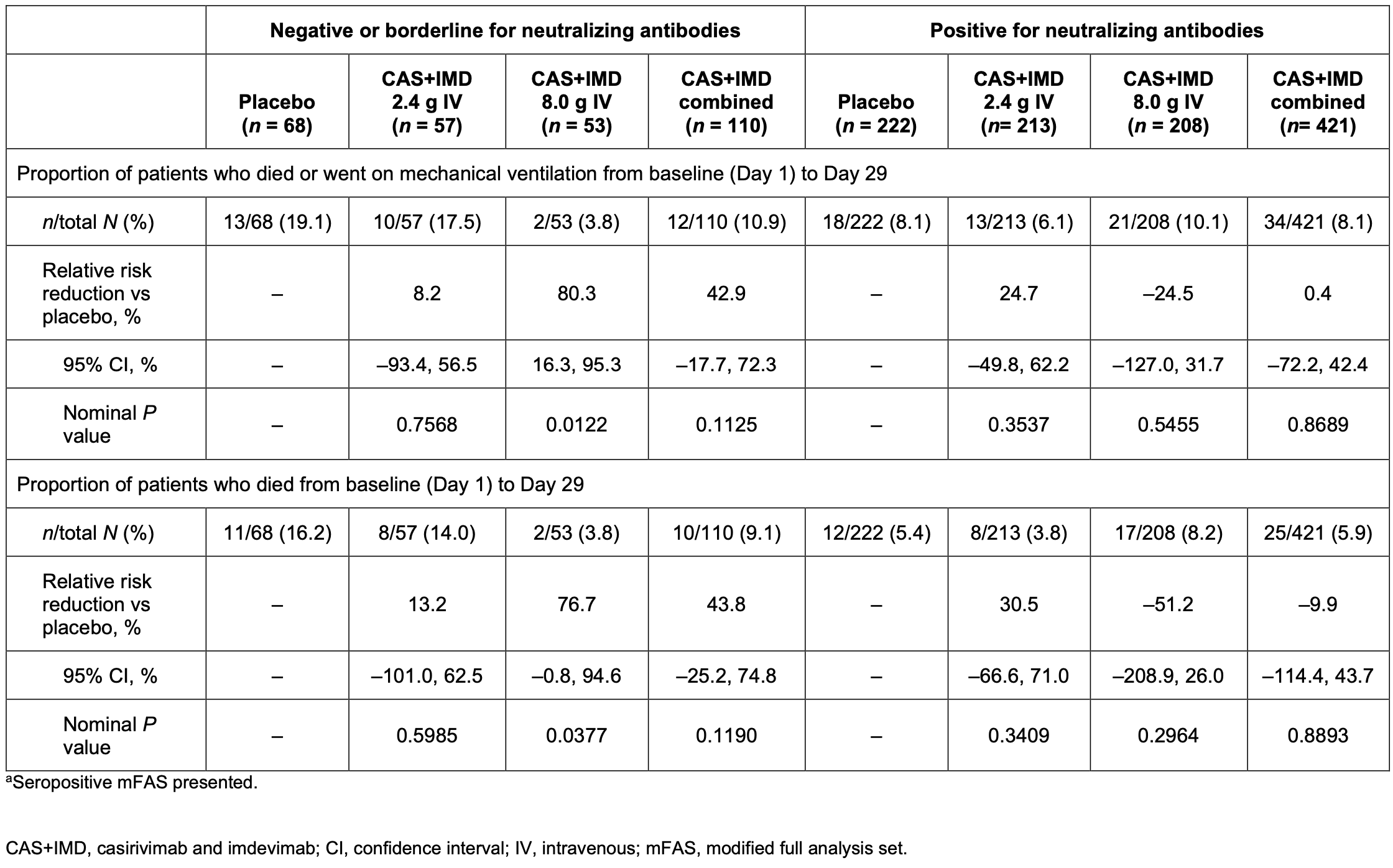
Post hoc analysis of a phase 1/2/3 RCT1 of 864 seropositive hospitalized COVID-19 patients showing casirivimab/imdevimab reduced viral load, death/mechanical ventilation, and all-cause mortality in the 20% of patients who were negative/borderline for neutralizing antibodies at baseline, but not in patients positive for neutralizing antibodies.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants2-8.
|
risk of death, 16.9% lower, RR 0.83, p = 0.48, treatment 35 of 531 (6.6%), control 23 of 290 (7.9%), NNT 75, day 28, mFAS.
|
|
risk of death/intubation, 19.0% lower, RR 0.81, p = 0.38, treatment 46 of 531 (8.7%), control 31 of 290 (10.7%), NNT 49, day 28, mFAS.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Somersan-Karakaya et al., Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19, The Journal of Infectious Diseases, doi:10.1093/infdis/jiac320.
2.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
3.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
4.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
5.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
6.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Hooper et al., 20 Dec 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 14 authors, average treatment delay 6.0 days, trial NCT04426695 (history).
Contact: andrea.hooper@regeneron.com.
Casirivimab and Imdevimab Treatment Reduces Viral Load and Improves Clinical Outcomes in Seropositive Hospitalized COVID-19 Patients with Nonneutralizing or Borderline Neutralizing Antibodies
mBio, doi:10.1128/mbio.01699-22
We conducted a post hoc analysis in seropositive patients who were negative or borderline for functional neutralizing antibodies (NAbs) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at baseline from a phase 1, 2, and 3 trial of casirivimab and imdevimab (CAS1IMD) treatment in hospitalized coronavirus disease 2019 (COVID-19) patients on low-flow or no supplemental oxygen prior to the emergence of Omicron-lineage variants. Patients were randomized to a single dose of 2.4 g CAS1IMD, 8.0 g CAS1IMD, or placebo. Patients seropositive for anti-SARS-CoV-2 antibodies at baseline were analyzed by their baseline neutralizing antibody status. At baseline, 20.6% (178/864) of seropositive patients were negative or borderline for neutralizing antibodies, indicating negative or very low functionally neutralizing anti-SARS-CoV-2 antibodies. CAS1IMD reduced viral load in patients who were negative or borderline for neutralizing antibodies versus placebo, but not in patients who were positive for neutralizing antibodies. In patients who were negative or borderline for neutralizing antibodies, we observed a trend in reduction of the proportion of patients who died or required mechanical ventilation, as well as in all-cause mortality, by day 29 with CAS1IMD versus placebo. The proportions of patients who died or required mechanical ventilation from days 1 to 29 were 19.1% in the placebo group and 10.9% in the CAS1IMD combined-dose group, and the proportions of patients who died (all-cause mortality) from days 1 to 29 were 16.2% in the placebo group and 9.1% in the CAS1IMD combined-dose group. In patients who were positive for neutralizing antibodies, no measurable harm or benefit was observed in either the proportion of patients who died or required mechanical ventilation or the proportion of patients who died (all-cause mortality). In hospitalized COVID-19 patients on low-flow or no supplemental oxygen, CAS1IMD reduced viral load, the risk of death or mechanical ventilation, and all-cause mortality in seropositive patients who were negative or borderline for neutralizing antibodies. IMPORTANCE The clinical benefit of CAS1IMD in hospitalized seronegative patients with COVID-19 has previously been demonstrated, although these studies observed no clinical benefit in seropositive patients. As the prevalence of SARS-CoV-2-seropositive individuals rises due to both vaccination and previous infection, it is important to understand whether there is a subset of hospitalized patients with COVID-19 with antibodies against SARS-CoV-2 who could benefit from anti-SARS-CoV-2 monoclonal antibody treatment. This post hoc analysis demonstrates that there is a subset of hospitalized seropositive patients with inadequate SARS-CoV-2-neutralizing antibodies (i.e., those who were
References
Andrews, Tessier, Stowe, Gower, Kirsebom et al., Duration of protection against mild and severe disease by Covid-19 vaccines, N Engl J Med, doi:10.1056/NEJMoa2115481
Bernal, Andrews, Gower, Gallagher, Simmons et al., Effectiveness of Covid-19 vaccines against the B.1 .617.2 (Delta) variant, N Engl J Med, doi:10.1056/NEJMoa2108891
Hansen, Baum, Pascal, Russo, Giordano et al., Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science, doi:10.1126/science.abd0827
Lundgren, Grund, Barkauskas, Holland, Gottlieb et al., Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels, Ann Intern Med, doi:10.7326/M21-3507
Montejano, Marcelo, Falces-Romero, Del Valle, Soto et al., Efficacy of sotrovimab for persistent coronavirus disease-2019 in a severely immunocompromised person living with HIV, AIDS, doi:10.1097/QAD.0000000000003179
Nordstrom, Ballin, Nordstrom, Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden, Lancet, doi:10.1016/S0140-6736(22)00089-7
O'brien, Forleo-Neto, Musser, Chan, Sarkar et al., Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med, doi:10.1056/NEJMoa2109682
Somersan-Karakaya, Mylonakis, Menon, Wells, Ali et al., Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19, J Infect Dis, doi:10.1093/infdis/jiac320
Stadler, Chai, Schlub, Cromer, Polizzotto et al., Determinants of passive antibody effectiveness in SARS-CoV-2 infection, doi:10.1101/2022.03.21.22272672
Stein, Oviedo-Orta, Kampman, Mcginniss, Betts et al., Compassionate use of REGEN-COV in patients with COVID-19 and immunodeficiency-associated antibody disorders, Clin Infect Dis, doi:10.1093/cid/ciab1059
Thomas, Moreira, Kitchin, Absalon, Gurtman et al., Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months, N Engl J Med, doi:10.1056/NEJMoa2110345
Vandergaast, Carey, Reiter, Lathrum, Lech et al., IMMUNO-COV v2.0: development and validation of a high-throughput clinical assay for measuring SARS-CoV-2-neutralizing antibody titers, NAb Status mBio
Vandergaast, Carey, Reiter, Lech, Gnanadurai et al., Development and validation of IMMUNO-COV: a high-throughput clinical assay for detecting antibodies that neutralize SARS-CoV-2, doi:10.1101/2020.05.26.117549
Ward, Cooke, Whitaker, Redd, Eales et al., REACT-2 round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England, doi:10.1101/2021.02.26.21252512
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
DOI record:
{
"DOI": "10.1128/mbio.01699-22",
"ISSN": [
"2150-7511"
],
"URL": "http://dx.doi.org/10.1128/mbio.01699-22",
"abstract": "<jats:p>The clinical benefit of CAS+IMD in hospitalized seronegative patients with COVID-19 has previously been demonstrated, although these studies observed no clinical benefit in seropositive patients. As the prevalence of SARS-CoV-2-seropositive individuals rises due to both vaccination and previous infection, it is important to understand whether there is a subset of hospitalized patients with COVID-19 with antibodies against SARS-CoV-2 who could benefit from anti-SARS-CoV-2 monoclonal antibody treatment.</jats:p>",
"alternative-id": [
"10.1128/mbio.01699-22"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-06-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-09-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-10-18"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1131-1229",
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"authenticated-orcid": true,
"family": "Hooper",
"given": "Andrea T.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Somersan-Karakaya",
"given": "Selin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "McCarthy",
"given": "Shane E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4624-0777",
"affiliation": [
{
"name": "Brown University, Providence, Rhode Island, USA"
}
],
"authenticated-orcid": true,
"family": "Mylonakis",
"given": "Eleftherios",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Ali",
"given": "Shazia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Mei",
"given": "Jingning",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Bhore",
"given": "Rafia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Mahmood",
"given": "Adnan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Geba",
"given": "Gregory P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Dakin",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Weinreich",
"given": "David M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Yancopoulos",
"given": "George D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Herman",
"given": "Gary A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA"
}
],
"family": "Hamilton",
"given": "Jennifer D.",
"sequence": "additional"
}
],
"container-title": "mBio",
"container-title-short": "mBio",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2022,
10,
18
]
],
"date-time": "2022-10-18T13:15:16Z",
"timestamp": 1666098916000
},
"deposited": {
"date-parts": [
[
2022,
12,
20
]
],
"date-time": "2022-12-20T14:16:36Z",
"timestamp": 1671545796000
},
"editor": [
{
"affiliation": [],
"family": "Miller",
"given": "Matthew S.",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T00:54:43Z",
"timestamp": 1714524883376
},
"is-referenced-by-count": 3,
"issue": "6",
"issued": {
"date-parts": [
[
2022,
12,
20
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2022,
12,
20
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
20
]
],
"date-time": "2022-12-20T00:00:00Z",
"timestamp": 1671494400000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
20
]
],
"date-time": "2022-12-20T00:00:00Z",
"timestamp": 1671494400000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/mbio.01699-22",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/mbio.01699-22",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2022,
12,
20
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
20
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"DOI": "10.1056/NEJMoa2115481",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_2_2"
},
{
"DOI": "10.1056/NEJMoa2108891",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_3_2"
},
{
"DOI": "10.1056/NEJMoa2110345",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_4_2"
},
{
"DOI": "10.1101/2021.02.26.21252512",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_5_2"
},
{
"DOI": "10.1101/2022.03.21.22272672",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_6_2"
},
{
"DOI": "10.1093/cid/ciab1059",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_7_2"
},
{
"DOI": "10.1097/QAD.0000000000003179",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_8_2"
},
{
"DOI": "10.1126/science.abd0827",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_9_2"
},
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_10_2"
},
{
"DOI": "10.1056/NEJMoa2109682",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_11_2"
},
{
"DOI": "10.1016/S0140-6736(22)00163-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_12_2"
},
{
"DOI": "10.1093/infdis/jiac320",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_13_2"
},
{
"key": "e_1_3_2_14_2",
"unstructured": "Centers for Disease Control and Prevention. Nationwide COVID-19 infection- and vaccination-induced antibody seroprevalence (blood donations). https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence. Accessed 22 March 2022."
},
{
"key": "e_1_3_2_15_2",
"unstructured": "Regeneron Pharmaceuticals Inc. 2021. Regeneron’s next generation monoclonal antibodies are active against all known variants of concern including both Omicron and Delta. https://investor.regeneron.com/static-files/4aed42a1-3d26-48af-bd01-3f0c92938c11. Accessed 10 March 2022."
},
{
"key": "e_1_3_2_16_2",
"unstructured": "U.S. Food and Drug Administration. 2021. Fact sheet for health care providers. Emergency use authorization (EUA) of REGEN-COV (casirivimab and imdevimab). https://www.fda.gov/media/145611/download. Accessed 24 June 2021."
},
{
"DOI": "10.1101/2020.05.26.117549",
"doi-asserted-by": "crossref",
"key": "e_1_3_2_17_2",
"unstructured": "Vandergaast R Carey T Reiter S Lech P Gnanadurai C Tesfay M Buehler J Suksanpaisan L Naik S Brunton B Recker J Haselton M Ziegler C Roesler A Mills JR Theel E Weaver SC Rafael G Roforth MM Jerde C Tran S Diaz RM Bexon A Baum A Kyratsous CA Peng KW Russell SJ. 2020. Development and validation of IMMUNO-COV: a high-throughput clinical assay for detecting antibodies that neutralize SARS-CoV-2. bioRxiv doi:10.1101/2020.05.26.117549."
},
{
"DOI": "10.7326/M21-3507",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_18_2"
},
{
"key": "e_1_3_2_19_2",
"unstructured": "U.S. Food and Drug Administration. 2022. Emergency use authorization 091. https://www.fda.gov/media/145610/download. Accessed 10 February 2022."
},
{
"DOI": "10.1016/S0140-6736(22)00089-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_20_2"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_21_2"
},
{
"DOI": "10.1128/mSphere.00170-21",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_22_2"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2022.06.14.22276389",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/mbio.01699-22"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Casirivimab and Imdevimab Treatment Reduces Viral Load and Improves Clinical Outcomes in Seropositive Hospitalized COVID-19 Patients with Nonneutralizing or Borderline Neutralizing Antibodies",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "13"
}
