The effects of probiotic Lactobacillus acidophilus and colchicine on the control of symptoms, duration, and disease progression of mild and moderate cases of COVID-19: A randomized controlled clinical trial
et al., Research Square, doi:10.21203/rs.3.rs-3049708/v1, Jun 2023
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 150 patients in Egypt showing no significant difference in outcomes with colchicine. SOC included vitamin C, D, and zinc. Colchicine 0.5mg tid days 1-3, bid days 4-7.
Study covers colchicine and probiotics.
|
risk of hospitalization, 40.0% higher, RR 1.40, p = 0.76, treatment 7 of 50 (14.0%), control 5 of 50 (10.0%).
|
|
risk of no recovery, 3.6% lower, RR 0.96, p = 1.00, treatment 27 of 50 (54.0%), control 28 of 50 (56.0%), NNT 50.
|
|
recovery time, no change, relative time 1.00, treatment 50, control 50.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hassan et al., 13 Jun 2023, Randomized Controlled Trial, Egypt, preprint, 6 authors, study period July 2021 - August 2022.
Contact: dr.samar.osama@med.asu.edu.eg.
The effects of probiotic Lactobacillus acidophilus and colchicine on the control of symptoms, duration, and disease progression of mild and moderate cases of COVID-19: A randomized controlled clinical trial
doi:10.21203/rs.3.rs-3049708/v1
Background Coronavirus disease 2019 (COVID-19) is a newly emerging human disease caused by a novel coronavirus, causing a global pandemic crisis. Probiotics and/or colchicine may be considered as options for treatment since they have anti-viral, anti-in ammatory, and immunomodulatory effects.
Objective To assess the effectiveness of probiotic supplements (Lactobacillus acidophilus) and colchicine on symptoms, duration, and progression of mild and moderate cases of COVID-19 infection.
Methods A three-arm randomized controlled clinical trial was carried out in the triage clinic of the family medicine department at Ain Shams University Hospitals on 150 participants who had been diagnosed as COVID-19 patients with mild and moderate severity. Patients aged below 18 years or above 65 years with any comorbidities, pregnant or lactating females, and severe COVID-19 con rmed cases were excluded. Randomization was done by using sealed envelopes containing codes for intervention or control. Patients are followed up for improvement of their symptoms with no development of new symptoms over the course of two weeks.
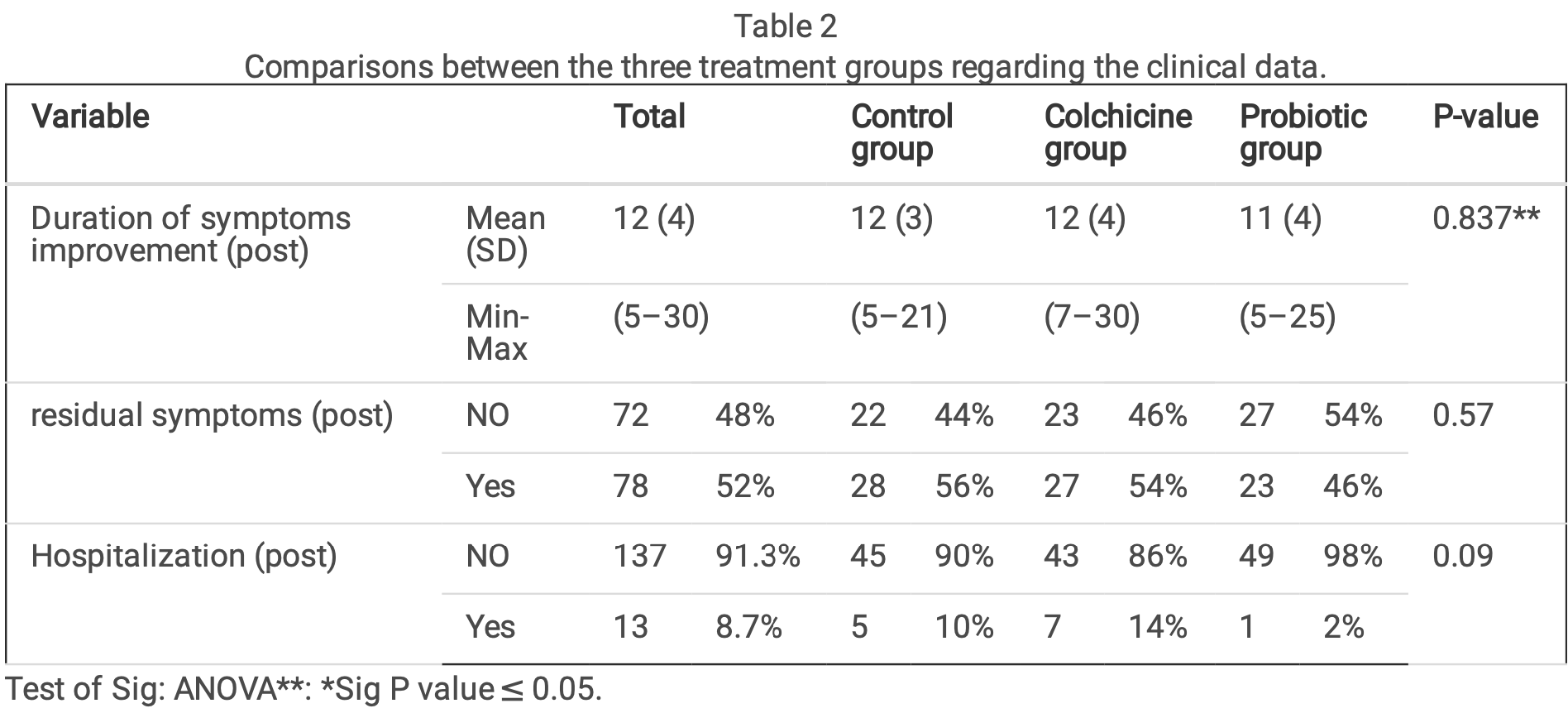
Results A total of 150 patients with mild and moderate severity of COVID-19 were enrolled in the study, 50 patients in each arm; around one third (34.7%) of the participants were aged between 29 and 39 years; one-quarter (24.7%) were aged between 18 and 28 years and 40.6% were aged 40 years and above. The mean duration of symptoms improvement was 12, 11 and 12 in the colchicine, probiotic, and control groups, respectively. Improvement of in ammatory markers over time occurred in each of the three groups, with no statistically signi cant difference between them.
Conclusion Probiotic Lactobacillus acidophilus and colchicine shows no signi cant effect on the symptoms, duration, and progression of mild and moderate cases of COVID-19.
Declarations Author contributions: Each author declares having participated in the activities.
Funding: None. Figure 1 See image above for gure legend.
References
Abdelfattah, Korra, Ahmed, Use of colchicine in COVID-19 hospitalized patients, Egypt J Chest Dis Tuberc, doi:10.4103/ecdt.ecdt_59_21
Angurana, Coronavirus disease 2019: think about the link, Br J Nutr, doi:10.1017/S000711452000361X
Darbandi, Asadi, Ghanavati, Emamie, The effect of probiotics on respiratory tract infection with special emphasis on COVID-19: Systemic review 2010-20, Int J Infect Dis, doi:10.1016/j.ijid.2021.02.011
Deftereos, Giannopoulos, Vrachatis, Siasos, Sg, Effect of vs Standard Care on Cardiac and In ammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13136
Doerre, The uence of gender on COVID-19 infections and mortality in Germany: Insights from age-and gender-speci c modeling of contact rates, infections, and deaths in the early phase of the pandemic, PLoS One, doi:10.1371/journal.pone.0268119
Dorward, Yu, Hayward, Saville, Gbinigie et al., Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, Br J Gen Pract, doi:10.3399/BJGP.2022.0083
Effenberger, Grabherr, Mayr, Schwaerzler, Nairz et al., Faecal calprotectin indicates intestinal in ammation in COVID-19, Gut, doi:10.1136/gutjnl-2020-321388
Farsalinos, Bagos, Giannouchos, Niaura, Barbouni et al., Smoking prevalence among hospitalized COVID-19 patients and its association with disease severity and mortality: an expanded reanalysis of a recent publication, Harm Reduct J, doi:10.1186/s12954-020-00437-5
Gorial, Maulood, Abdulamir, Alnuaimi, Bonyan, Randomized controlled trial of colchicine add on to the standard therapy in moderate and severe corona virus Disease-19 infection, Ann Med Surg, doi:10.1016/j.amsu.2022.103593
Hakki, Zhou, Jonnerby, Singanayagam, Barnett et al., Onset and of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study, Lancet Respir Med, doi:10.1016/S2213-2600(22)00226-0
Hariyanto, Halim, Jodhinata, Yanto, Kurniawan, Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, Clin Exp Pharmacol Physiol, doi:10.1111/1440-1681.13488
Hill, Guarner, Reid, Gibson, Merenstein et al., Expert consensus document. The International Scienti c Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic, Nat Rev Gastroenterol Hepatol, doi:10.1038/nrgastro.2014.66
Karatza, Ismailos, Karalis Colchicine for the treatment of COVID-19 patients: e cacy, safety, and model informed dosage regimens, Xenobiotica, doi:10.1080/00498254.2021
Leung, Hui, Kraus, Colchicine-Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum, doi:10.1016/j.semarthrit.2015.06.013
Lopes, Bonjorno, Giannini, Amaral, Menezes et al., Bene cial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open, doi:10.1136/rmdopen-2020-001455
Qin, Zhou, Hu, Zhang, Yang et al., Dysregulation of Immune Response in Patients with, Clin Infect Dis, doi:10.1093/cid/ciaa248
Sandhu, Tieng, Chilimuri, Franchin, Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection, Can J Infect Dis Med Microbiol, doi:10.1155/2020/8865954
Sarwar, Ali, Fatima, Sarfraz, Sarfraz et al., COVID-19, and hematological parameters: A meta-analysis, J Clin Lab Anal, doi:10.1002/jcla.24057
Siemieniuk, Bartoszko, Zeraatkar, Qasim, Martinez, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.m2980
Soraya, Ulhaq, Crucial laboratory parameters in COVID-19 diagnosis and prognosis: An updated meta-analysis, Med Clin (Barc), doi:10.1016/j.medcli.2020.05.017
Tardif, Bouabdallaoui, Allier, Gaudet, Shah et al., Colchicine for communitytreated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebocontrolled, multicentre trial, Lancet Respir Med, doi:10.1016/S2213-2600
Teranaka, Pan, Discharge criteria for patients with COVID-19 to long-term care facilities requires modi cation, Clin Med, doi:10.7861/clinmed.Let.21.1.2
Terkeltaub, Furst, Bennett, Kook, Crockett et al., High versus low dosing of oral colchicine for early acute gout are: Twenty-four-hour outcome of the rst multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study, Arthritis Rheum, doi:10.1002/art.27327
Wischmeyer, Tang, Ren, Bohannon, Ramirez et al., Daily Lactobacillus probiotic versus placebo in household contacts (PROTECT-EHC): a randomized clinical trial, MedRxiv, doi:10.1101/2022.01.04.21268275
Yasmin, Najeeb, Moeed, Hassan, Asghar, Safety and e cacy of in COVID-19 patients: A systematic review and meta-analysis of randomized control trials, PLoS One, doi:10.1371/journal.pone.0266245
Yeo Kaushal, Yeo, Enteric involvement of coronaviruses: Is faecal-oral transmission of SARS-CoV-2 possible?, Lancet Gastroenterol Hepatol, doi:10.1016/S2468-1253(20)30048-0
Zhang, Yeh, Ding, Liu, Zhang et al., Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate, Synth Syst Biotechnol, doi:10.1016/j.synbio.2018.03.001
Zuo, Zhang, Lui, Yeoh, Li et al., Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization, Gastroenterology, doi:10.1053/j.gastro.2020.05.048
DOI record:
{
"DOI": "10.21203/rs.3.rs-3049708/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-3049708/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Background\n Coronavirus disease 2019 (COVID-19) is a newly emerging human disease caused by a novel coronavirus, causing a global pandemic crisis. Probiotics and/or colchicine may be considered as options for treatment since they have anti-viral, anti-inflammatory, and immunomodulatory effects.\nObjective\n To assess the effectiveness of probiotic supplements (Lactobacillus acidophilus) and colchicine on symptoms, duration, and progression of mild and moderate cases of COVID-19 infection.\nMethods\n A three-arm randomized controlled clinical trial was carried out in the triage clinic of the family medicine department at Ain Shams University Hospitals on 150 participants who had been diagnosed as COVID-19 patients with mild and moderate severity. Patients aged below 18 years or above 65 years with any co-morbidities, pregnant or lactating females, and severe COVID-19 confirmed cases were excluded. Randomization was done by using sealed envelopes containing codes for intervention or control. Patients are followed up for improvement of their symptoms with no development of new symptoms over the course of two weeks.\nResults\n A total of 150 patients with mild and moderate severity of COVID-19 were enrolled in the study, 50 patients in each arm; around one third (34.7%) of the participants were aged between 29 and 39 years; one-quarter (24.7%) were aged between 18 and 28 years and 40.6% were aged 40 years and above. The mean duration of symptoms improvement was 12, 11 and 12 in the colchicine, probiotic, and control groups, respectively. Improvement of inflammatory markers over time occurred in each of the three groups, with no statistically significant difference between them.\nConclusion\n Probiotic Lactobacillus acidophilus and colchicine shows no significant effect on the symptoms, duration, and progression of mild and moderate cases of COVID-19.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
6,
11
]
]
},
"author": [
{
"affiliation": [
{
"name": "Ain Shams University Hospital"
}
],
"family": "Hassan",
"given": "Samar Osama Ahmed",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Ain Shams University Hospital"
}
],
"family": "Hassan",
"given": "Ahmed Nour El-Din",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ain Shams University Hospital"
}
],
"family": "Mohamed",
"given": "Manal Sabry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ain Shams University Hospital"
}
],
"family": "Ashram",
"given": "Mohamed Nabil Badawy Al",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ain Shams University Hospital"
}
],
"family": "Nesim",
"given": "Mina Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ain Shams University Hospital"
}
],
"family": "Allam",
"given": "Mohamed Farouk",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T20:48:50Z",
"timestamp": 1686689330000
},
"deposited": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T20:48:56Z",
"timestamp": 1686689336000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2023,
6,
14
]
],
"date-time": "2023-06-14T04:22:00Z",
"timestamp": 1686716520236
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6,
13
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T00:00:00Z",
"timestamp": 1686614400000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-3049708/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-3049708/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
6,
13
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2023,
6,
13
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"key": "ref1",
"unstructured": "World Health Organization. Coronavirus disease (covid-19) - events as they happen.Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed on 2023 Apr 29."
},
{
"key": "ref2",
"unstructured": "Centres for Disease Control and Prevention. \"Coronavirus disease 2019 (COVID-19) 2021 case definition. Available from: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/ Accessed on 2023 Apr 29."
},
{
"DOI": "10.1017/S000711452000361X",
"article-title": "Coronavirus disease 2019: think about the link",
"author": "Angurana SK",
"doi-asserted-by": "publisher",
"first-page": "1564",
"issue": "10",
"journal-title": "Br J Nutr",
"key": "ref3",
"unstructured": "Angurana SK, Bansal A. Coronavirus disease 2019: think about the link. Br J Nutr. 2021 Nov;28(10):1564–70. 10.1017/S000711452000361X.",
"volume": "28"
},
{
"DOI": "10.1038/nrgastro.2014.66",
"article-title": "Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic",
"author": "Hill C",
"doi-asserted-by": "publisher",
"first-page": "506",
"issue": "8",
"journal-title": "Nat Rev Gastroenterol Hepatol",
"key": "ref4",
"unstructured": "Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014 Aug;11(8):506–14. 10.1038/nrgastro.2014.66.",
"volume": "11"
},
{
"DOI": "10.1016/S2468-1253(20)30048-0",
"article-title": "Enteric involvement of coronaviruses: Is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol",
"author": "Yeo C",
"doi-asserted-by": "publisher",
"first-page": "335",
"issue": "4",
"key": "ref5",
"unstructured": "Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: Is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020 Apr;5(4):335–7. doi: 10.1016/S2468-1253(20)30048-0.",
"volume": "5"
},
{
"DOI": "10.1136/gutjnl-2020-321388",
"article-title": "Faecal calprotectin indicates intestinal inflammation in COVID-19",
"author": "Effenberger M",
"doi-asserted-by": "publisher",
"first-page": "1543",
"issue": "8",
"journal-title": "Gut",
"key": "ref6",
"unstructured": "Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, Seiwald S, Scholl-Buergi S, Fritsche G, Bellmann-Weiler R, Weiss G, Müller T, Adolph TE, Tilg H. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020 Aug;69(8):1543–4. 10.1136/gutjnl-2020-321388.",
"volume": "69"
},
{
"DOI": "10.1016/j.synbio.2018.03.001",
"author": "Zhang H",
"doi-asserted-by": "publisher",
"key": "ref7",
"unstructured": "Zhang H, Yeh C, Jin Z, Ding L, Liu BY, Zhang L, Dannelly HK. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018 Mar 12;3(2):113–120. doi: 10.1016/j.synbio.2018.03.001."
},
{
"DOI": "10.1053/j.gastro.2020.05.048",
"article-title": "Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization",
"author": "Zuo T",
"doi-asserted-by": "publisher",
"first-page": "944",
"issue": "3",
"journal-title": "Gastroenterology",
"key": "ref8",
"unstructured": "Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020 Sep;159(3):944–955e8. 10.1053/j.gastro.2020.05.048.",
"volume": "159"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"article-title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial",
"author": "Lopes MI",
"doi-asserted-by": "publisher",
"first-page": "e001455",
"issue": "1",
"journal-title": "RMD Open",
"key": "ref9",
"unstructured": "Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, Dib SM, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021 Feb;7(1):e001455. 10.1136/rmdopen-2020-001455.",
"volume": "7"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"article-title": "Colchicine-Update on mechanisms of action and therapeutic uses",
"author": "Leung YY",
"doi-asserted-by": "publisher",
"first-page": "341",
"issue": "3",
"journal-title": "Semin Arthritis Rheum",
"key": "ref10",
"unstructured": "Leung YY, Yao Hui LL, Kraus VB. Colchicine-Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015 Dec;45(3):341–50. 10.1016/j.semarthrit.2015.06.013.",
"volume": "45"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif J-C",
"doi-asserted-by": "publisher",
"first-page": "924",
"issue": "8",
"journal-title": "Lancet Respir Med",
"key": "ref11",
"unstructured": "Tardif J-C, Bouabdallaoui N, L'Allier PL, Gaudet D, Shah B, Pillinger MH, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021 Aug;9(8):924–32. 10.1016/S2213-2600(21)00222-8.",
"volume": "9"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk RAC",
"doi-asserted-by": "publisher",
"first-page": "m2980",
"journal-title": "BMJ 2020 Jul",
"key": "ref12",
"unstructured": "Siemieniuk RAC, Bartoszko JJ, Zeraatkar D, Kum E, Qasim A, Díaz Martinez JP et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ 2020 Jul 30;370:m2980. doi: 10.1136/bmj.m2980.",
"volume": "30;370"
},
{
"DOI": "10.1080/00498254.2021",
"article-title": "Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens",
"author": "Karatza E",
"doi-asserted-by": "publisher",
"first-page": "643",
"issue": "6",
"journal-title": "Xenobiotica",
"key": "ref13",
"unstructured": "Karatza E, Ismailos G, Karalis V. Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens. Xenobiotica. 2021 Jun;51(6):643–56. 10.1080/00498254.2021.",
"volume": "51"
},
{
"DOI": "10.7861/clinmed. Let.21.1.2",
"article-title": "Discharge criteria for patients with COVID-19 to long-term care facilities requires modification",
"author": "Teranaka W",
"doi-asserted-by": "publisher",
"first-page": "e116",
"issue": "1",
"journal-title": "Clin Med (Lond)",
"key": "ref14",
"unstructured": "Teranaka W, Pan D. Discharge criteria for patients with COVID-19 to long-term care facilities requires modification. Clin Med (Lond). 2021 Jan;21(1):e116–7. 10.7861/clinmed. Let.21.1.2.",
"volume": "21"
},
{
"DOI": "10.1371/journal.pone.0268119",
"author": "Doerre A",
"doi-asserted-by": "publisher",
"key": "ref15",
"unstructured": "Doerre A, Doblhammer G. The influence of gender on COVID-19 infections and mortality in Germany: Insights from age- and gender-specific modeling of contact rates, infections, and deaths in the early phase of the pandemic. PLoS One. 2022 May 6;17(5): e0268119. doi: 10.1371/journal.pone.0268119."
},
{
"DOI": "10.1186/s12954-020-00437-5",
"article-title": "Smoking prevalence among hospitalized COVID-19 patients and its association with disease severity and mortality: an expanded re-analysis of a recent publication",
"author": "Farsalinos K",
"doi-asserted-by": "publisher",
"first-page": "9",
"issue": "1",
"journal-title": "Harm Reduct J 2021 Jan",
"key": "ref16",
"unstructured": "Farsalinos K, Bagos PG, Giannouchos T, Niaura R, Barbouni A, Poulas K. Smoking prevalence among hospitalized COVID-19 patients and its association with disease severity and mortality: an expanded re-analysis of a recent publication. Harm Reduct J 2021 Jan 16;18(1):9. doi: 10.1186/s12954-020-00437-5.",
"volume": "16"
},
{
"DOI": "10.1016/S2213-2600(22)00226-0",
"article-title": "Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study",
"author": "Hakki S",
"doi-asserted-by": "publisher",
"first-page": "1061",
"issue": "11",
"journal-title": "Lancet Respir Med",
"key": "ref17",
"unstructured": "Hakki S, Zhou J, Jonnerby J, Singanayagam A, Barnett JL, Madon KJ, et al. Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study. Lancet Respir Med. 2022 Nov;10(11):1061–73. 10.1016/S2213-2600(22)00226-0.",
"volume": "10"
},
{
"key": "ref18",
"unstructured": "Centres for Disease Control and Prevention. SARS-COV-2 variant classifications and definitions; Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. Accessed on 2023 Apr 29."
},
{
"DOI": "10.1016/j.amsu.2022.103593",
"article-title": "Randomized controlled trial of colchicine add on to the standard therapy in moderate and severe corona virus Disease-19 infection",
"author": "Gorial FI",
"doi-asserted-by": "publisher",
"first-page": "103593",
"journal-title": "Ann Med Surg (Lond)",
"key": "ref19",
"unstructured": "Gorial FI, Maulood MF, Abdulamir AS, Alnuaimi AS, abdulrrazaq MK, Bonyan FA. Randomized controlled trial of colchicine add on to the standard therapy in moderate and severe corona virus Disease-19 infection. Ann Med Surg (Lond). 2022 May;77:103593. 10.1016/j.amsu.2022.103593.",
"volume": "77"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"author": "Dorward J",
"doi-asserted-by": "publisher",
"key": "ref20",
"unstructured": "Dorward J, Yu L-M, Hayward G, Saville BR, Gbinigie O, Van Hecke O et al. Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br J Gen Pract. 2022 Jun 30;72(720): e446-e455. doi: 10.3399/BJGP.2022.0083."
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"doi-asserted-by": "crossref",
"key": "ref21",
"unstructured": "Colchicine in patients admitted to hospital with covid-19 (recovery): Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021 Dec;9(12):1419–1426. doi: 10.1016/S2213-2600(21)00435-5."
},
{
"DOI": "10.1111/1440-1681.13488",
"article-title": "Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis",
"author": "Hariyanto TI",
"doi-asserted-by": "publisher",
"first-page": "823",
"issue": "6",
"journal-title": "Clin Exp Pharmacol Physiol",
"key": "ref22",
"unstructured": "Hariyanto TI, Halim DA, Jodhinata C, Yanto TA, Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021 Jun;48(6):823–30. 10.1111/1440-1681.13488.",
"volume": "48"
},
{
"DOI": "10.4103/ecdt.ecdt_59_21",
"author": "AbdelFattah EB",
"doi-asserted-by": "publisher",
"key": "ref23",
"unstructured": "AbdelFattah EB, Korra EEA, Ahmed MA. Use of colchicine in COVID-19 hospitalized patients. Egypt J Chest Dis Tuberc 2022; 71:290-5. 2022;71(3):290. doi: 10.4103/ecdt.ecdt_59_21."
},
{
"DOI": "10.1002/art.27327",
"article-title": "High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study",
"author": "Terkeltaub RA",
"doi-asserted-by": "publisher",
"first-page": "1060",
"issue": "4",
"journal-title": "Arthritis Rheum 2010",
"key": "ref24",
"unstructured": "Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum 2010 Apr;62(4):1060–8. doi: 10.1002/art.27327.",
"volume": "62"
},
{
"DOI": "10.1371/journal.pone.0266245",
"author": "Yasmin F",
"doi-asserted-by": "publisher",
"key": "ref25",
"unstructured": "Yasmin F, Najeeb H, Moeed A, Hassan W, Khatri M, Asghar MS et al. Safety and efficacy of colchicine in COVID-19 patients: A systematic review and meta-analysis of randomized control trials. PLoS One. 2022 Apr 5;17(4): e0266245. doi: 10.1371/journal.pone.0266245."
},
{
"DOI": "10.1093/cid/ciaa248",
"doi-asserted-by": "crossref",
"key": "ref26",
"unstructured": "Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., … Tian, D. S. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020 Jul 28;71(15):762–768. doi: 10.1093/cid/ciaa248."
},
{
"DOI": "10.1016/j.medcli.2020.05.017",
"author": "Soraya GV",
"doi-asserted-by": "publisher",
"key": "ref27",
"unstructured": "Soraya GV, Ulhaq ZS. Crucial laboratory parameters in COVID-19 diagnosis and prognosis: An updated meta-analysis. Med Clin (Barc). 2020 Aug 28;155(4):143–151. doi: 10.1016/j.medcli.2020.05.017.",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"author": "Deftereos SG",
"doi-asserted-by": "publisher",
"key": "ref28",
"unstructured": "Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized with Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020 Jun 1;3(6): e2013136. doi: 10.1001/jamanetworkopen.2020.13136."
},
{
"DOI": "10.1002/jcla.24057",
"article-title": "COVID-19, and hematological parameters: A meta-analysis",
"author": "Sarwar M",
"doi-asserted-by": "publisher",
"first-page": "e24057",
"issue": "12",
"journal-title": "J Clin Lab Anal",
"key": "ref29",
"unstructured": "Sarwar M, Ali Z, Fatima M, Sarfraz Z, Sarfraz A, Cherrez-Ojeda I, Colchicine. COVID-19, and hematological parameters: A meta-analysis. J Clin Lab Anal. 2021 Dec;35(12):e24057. 10.1002/jcla.24057. Epub 2021 Oct 28.",
"volume": "35"
},
{
"DOI": "10.1155/2020/8865954",
"article-title": "Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection",
"author": "Sandhu T",
"doi-asserted-by": "publisher",
"first-page": "8865954",
"journal-title": "Can J Infect Dis Med Microbiol 2020 Oct",
"key": "ref30",
"unstructured": "Sandhu T, Tieng A, Chilimuri S, Franchin G. Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can J Infect Dis Med Microbiol 2020 Oct. 2020;27:8865954. 10.1155/2020/8865954.",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1101/2022.01.04.21268275",
"doi-asserted-by": "crossref",
"key": "ref31",
"unstructured": "Wischmeyer, P. E., Tang, H., Ren, Y., Bohannon, L., Ramirez, Z. E., Andermann, T.M., … Sung, A. D. (2022). Daily Lactobacillus probiotic versus placebo in COVID-19-exposed household contacts(PROTECT-EHC): a randomized clinical trial. MedRxiv, 2022-01. doi: https://doi.org/10.1101/2022.01.04.21268275."
},
{
"DOI": "10.1016/j.ijid.2021.02.011",
"article-title": "The effect of probiotics on respiratory tract infection with special emphasis on COVID-19: Systemic review 2010-20",
"author": "Darbandi A",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "Int J Infect Dis 2021",
"key": "ref32",
"unstructured": "Darbandi A, Asadi A, Ghanavati R, Afifirad R, Darb Emamie A, kakanj M et al. The effect of probiotics on respiratory tract infection with special emphasis on COVID-19: Systemic review 2010-20. Int J Infect Dis 2021 Apr; 105:91–104. doi: 10.1016/j.ijid.2021.02.011.",
"volume": "105"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-3049708/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "The effects of probiotic Lactobacillus acidophilus and colchicine on the control of symptoms, duration, and disease progression of mild and moderate cases of COVID-19: A randomized controlled clinical trial",
"type": "posted-content"
}
hassan
