Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID‑19: A randomized double‑blind placebo‑controlled trial
et al., International Journal of Molecular Medicine, doi:10.3892/ijmm.2022.5084, MEFECOVID-19, RPCEC00000388, Jan 2022
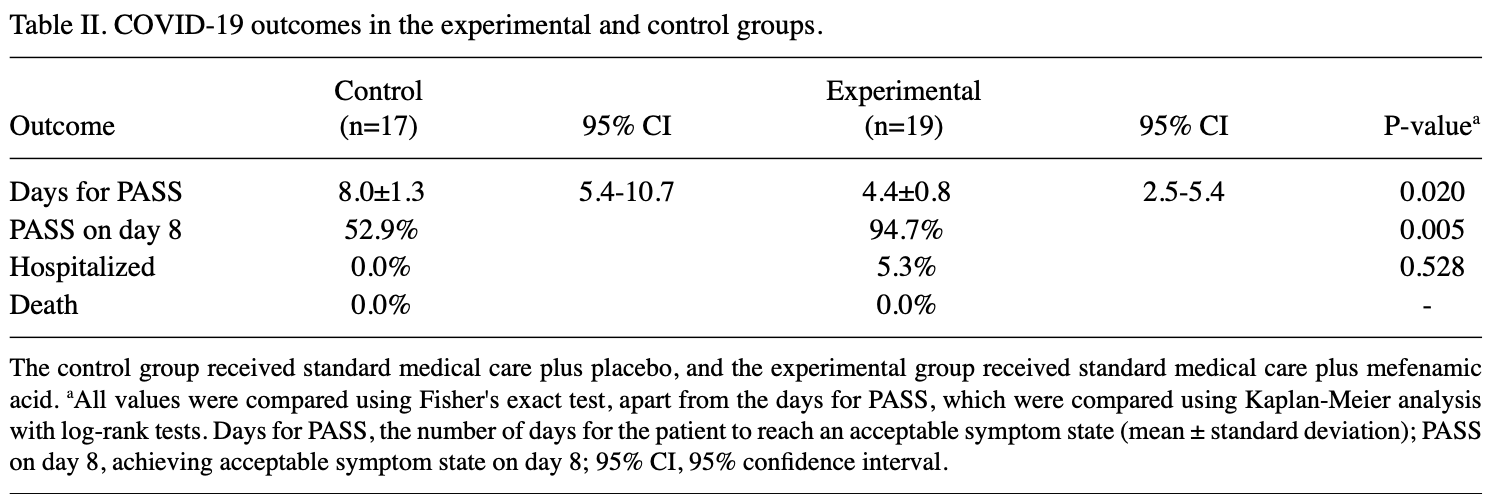
RCT 36 COVID-19 outpatients showing reduced time to an "acceptable symptom state" with mefenamic acid treatment, however the effect was driven by pain-related symptoms and is likely, at least in part, due to mefenamic acid’s analgesic action.
|
risk of hospitalization, 189.5% higher, RR 2.89, p = 1.00, treatment 1 of 19 (5.3%), control 0 of 17 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
PASS@8, 93.6% lower, relative time 0.06, p = 0.03, treatment 19, control 17, inverted to make RR<1 favor treatment, day 8.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Guzman‑Esquivel et al., 14 Jan 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Mexico, peer-reviewed, mean age 39.5, 20 authors, study period August 2020 - December 2020, average treatment delay 3.2 days, trial RPCEC00000388 (MEFECOVID-19).
Contact: ivan_delgado_enciso@ucol.mx.
Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID‑19: A randomized double‑blind placebo‑controlled trial
International Journal of Molecular Medicine, doi:10.3892/ijmm.2022.5084
Mefenamic acid is a non-steroidal anti-inflammatory drug exhibiting a wide range of anti-inflammatory, antipyretic, analgesic and probable antiviral activities. The present study evaluated the efficacy of treatment with mefenamic acid combined with standard medical care vs. standard medical care plus a placebo in ambulatory patients with coronavirus disease 2019 (COVID-19; nasal/oropharyngeal swabs reverse transcription-PCR test results positive for severe acute respiratory syndrome coronavirus 2). The present study is a phase II prospective, two-arm, parallel-group, randomized, double-blind placebo-controlled clinical trial which analyzed 36 patients. Two aspects were evaluated during the 14-day follow-up period: i) The time for reaching a patient acceptable symptom state (PASS), and ii) the last day of each COVID-19 symptom presentation. Adverse effects were evaluated. The clinical severity for all the patients in the study was mild (88.9%) and moderate (11.1%). The control (placebo) group achieved PASS on day 8.0±1.3, compared with day 4.4±0.8 in the mefenamic acid group (P= 0.020, Kaplan-Meier analyses using log-rank tests). Patients that received mefenamic acid plus standard medical care had a ~16-fold higher probability of achieving PASS on day 8 (adjusted RR, 15.57; 95% CI,; P= 0.035), compared with the placebo plus standard medical care group. All symptoms lasted for fewer days in the mefenamic acid group, compared with the placebo group; however, only the symptoms of headache (P= 0.008), retro-orbital eye pain (P= 0.049), and sore throat (P= 0.029) exhibited statistically significant differences. The experimental treatment produced no severe adverse effects. On the whole, the present study demonstrates that the administration of mefenamic acid markedly reduced the symptomatology and time to reach PASS in ambulatory patients with COVID-19. Due to its probable antiviral effects and potent anti-inflammatory mechanisms, mefenamic acid may prove to be useful in the treatment of cOVId-19, in combination with other drugs, including the new antivirals (remdesivir, molnupiravir, or favipiravir). However, future studies are also required to confirm these findings.
Authors' contributions JGE, IDE, HRGS, IPRS, JDE and MLMF designed the study and wrote the manuscript. HPGS, ACCV, JAGS, KAMR, CBCA, MWG, OBG, OGDE, HSGG and DCC visited and evaluated the patients. BAPM, FRL, VM and IDE designed and performed the statistical analysis. AGS and EMZ authorized and coordinated the recruitment of patients in the hospital. JDE and OGDE were the administrative coordinators of the clinical trial. IDE and HPGS confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate The present study was approved by the Ethics Committee of the Mexican Social Security Institute, delegation of the State of Colima, Mexico (July 29, 2020), and written informed consent was obtained from all the participants. All procedures performed in the present protocol were carried out in accordance with the Declaration of Helsinki and the clinical trial was registered as MEFECOVID-19: RPCEC00000388 in the Cuban Public Registry of Clinical Trials (RPCEC) database (August 31, 2021).
Patient consent for publication Not applicable.
Competing interests All authors declare that they have no competing interests.
References
Abdelnabi, Foo, Kaptein, Zhang, Do et al., The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model, EBioMedicine
Aggarwal, Chong, Sharma, Pillai, Naidu et al., Repurposing mefenamic acid in the management of covid-19, J Indian Med Assoc
Aggarwal, Mefenamic acid as steroid-sparing anti-inflammatory drug during viral phase of Covid-19: 5 case reports, Indian J Clin Pract
Alene, Yismaw, Assemie, Ketema, Mengist et al., Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis, PLoS One
Baby, Maity, Mehta, Suresh, Nayak et al., Targeting SARS-CoV-2 RNA-dependent RNA polymerase: An in silico drug repurposing for COVID-19, F1000Res
Basri, Ghani, Mahdy, Manaf, Ismail, Celecoxib versus mefenamic acid in the treatment of primary dysmenorrhea, Horm Mol Biol Clin Investig
Caldera-Villalobos, Garza-Veloz, Martínez-Avila, Delgado-Enciso, Ortiz-Castro et al., The coronavirus disease (COVID-19) challenge in Mexico: A critical and forced reflection as individuals and society, Front Public Health
Daniels, Rivers-Auty, Schilling, Spencer, Watremez et al., Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models, Nat Commun
Dehelean, Lazureanu, Coricovac, Mioc, Oancea et al., SARS-CoV-2: Repurposed drugs and novel therapeutic approaches-insights into chemical structure-biological activity and toxicological screening, J Clin Med
Delgado-Enciso, Garcia, Barajas-Saucedo, Mokay-Ramírez, Meza-Robles et al., Safety and efficacy of a COVID-19 treatment with nebulized and/or intravenous neutral electrolyzed saline combined with usual medical care vs. usual medical care alone: A randomized, open-label, controlled trial, Exp Ther Med
Etman, Farid, Nada, Ebian, In vitro/in vivo correlation of fast release mephenamic acid microspheres in humans, Med Princ Pract
Fagni, Simon, Tascilar, Schoenau, Sticherling et al., COVID-19 and immune-mediated inflammatory diseases: Effect of disease and treatment on COVID-19 outcomes and vaccine responses, Lancet Rheumatol
Freites-Martinez, Santana, Arias-Santiago, Viera, Using the common terminology criteria for adverse events (CTCAE-version 5.0) to evaluate the severity of adverse events of anticancer therapies, Actas Dermosifiliogr (Engl Ed)
Guzman-Esquivel, Mendoza-Hernandez, Jimenez, Avila-Zamora, Delgado-Enciso et al., decreased biochemical progression in patients with castration-resistant prostate cancer using a novel mefenamic acid anti-inflammatory therapy: A randomized controlled trial, Oncol Lett
Hu, Sun, Dai, Deng, Li et al., Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis, J Clin Virol
Kane, Sample size calculator
Kang, Chung, Yun, Lee, Lee et al., Inhibitory effects of anti-inflammatory drugs on interleukin-6 bioactivity, Biol Pharm Bull
Kelleni, Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes, Biomed Pharmacother
Manjili, Zarei, Habibi, Manjili, COVID-19 as an acute inflammatory disease, J Immunol
Melnikov, Tiburcio-Jimenez, Mendoza-Hernandez, Delgado-Enciso, De-Leon-Zaragoza et al., Improve cognitive impairment using mefenamic acid non-steroidal anti-inflammatory therapy: Additional beneficial effect found in a controlled clinical trial for prostate cancer therapy, Am J Transl Res
Moore, Bosco-Levy, Thurin, Blin, NSAIDs and COVID-19: A systematic review and meta-analysis, Drug Saf
Nitulescu, Paunescu, Moschos, Petrakis, Nitulescu et al., Comprehensive analysis of drugs to treat SARS-CoV-2 infection: Mechanistic insights into current COVID-19 therapies (Review), Int J Mol Med
Pincus, Swearingen, Bergman, Yazici, RAPID3 (routine assessment of patient index data 3), a rheumatoid arthritis index without formal joint counts for routine care: Proposed severity categories compared to disease activity score and clinical disease activity index categories, J Rheumatol
Rivett, Sridhar, Sparkes, Routledge, Jones et al., Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission, Elife
Rothan, Bahrani, Abdulrahman, Mohamed, Teoh et al., Mefenamic acid in combination with ribavirin shows significant effects in reducing chikungunya virus infection in vitro and in vivo, Antiviral Res
Rothan, Buckle, Ammar, Mohammadjavad, Shatrah et al., Study the antiviral activity of some derivatives of tetracycline and non-steroid anti inflammatory drugs towards dengue virus, Trop Biomed
Salepci, Turk, Ozcan, Bektas, Aybal et al., Symptomatology of COVID-19 from the otorhinolaryngology perspective: A survey of 223 SARS-CoV-2 RNA-positive patients, Eur Arch Otorhinolaryngol
Sherren, Ostermann, Agarwal, Meadows, Ioannou et al., COVID-19-related organ dysfunction and management strategies on the intensive care unit: A narrative review, Br J Anaesth
Shinkuma, Hamaguchi, Yamanaka, Mizuno, Correlation between dissolution rate and bioavailability of different commercial mefenamic acid capsules, Int J Pharm
Stancioiu, Papadakis, Kteniadakis, Izotov, Coleman et al., A dissection of SARS-CoV2 with clinical implications (Review), Int J Mol Med
Tenforde, Kim, Lindsell, Rose, Shapiro et al., Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, March-June 2020, MMWR Morb Mortal Wkly Rep
Tripathi, Kasture, Mefenamic acid: The evolution of a versatile NSAID, Indian J Clin Pract
Tsatsakis, Calina, Falzone, Petrakis, Mitrut et al., SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19, Food Chem Toxicol
Vivarelli, Falzone, Torino, Scandurra, Russo et al., Immune-checkpoint inhibitors from cancer to COVID-19: A promising avenue for the treatment of patients with COVID-19 (Review), Int J Oncol
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72314 cases from the Chinese center for disease control and prevention, JAMA
Zhao, Di, Xu, The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies, Cytokine Growth Factor Rev
DOI record:
{
"DOI": "10.3892/ijmm.2022.5084",
"ISSN": [
"1107-3756",
"1791-244X"
],
"URL": "http://dx.doi.org/10.3892/ijmm.2022.5084",
"article-number": "29",
"author": [
{
"affiliation": [
{
"name": "Department of Research, General Hospital of Zone No. 1 IMSS, Villa de Alvarez, Colima 28984, Mexico"
}
],
"family": "Guzman‑Esquivel",
"given": "Jose",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Galvan‑Salazar",
"given": "Hector",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Guzman‑Solorzano",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Cuevas‑Velazquez",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Guzman‑Solorzano",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Mokay‑Ramirez",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Paz‑Michel",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, General Hospital of Zone No. 1 IMSS, Villa de Alvarez, Colima 28984, Mexico"
}
],
"family": "Murillo‑Zamora",
"given": "Efren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, Foundation for Cancer Ethics, Education and Research of the Cancerology State Institute, Colima 28085, Mexico"
}
],
"family": "Delgado‑Enciso",
"given": "Josuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, General Hospital of Zone No. 1 IMSS, Villa de Alvarez, Colima 28984, Mexico"
}
],
"family": "Melnikov",
"given": "Valery",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Delgado‑Enciso",
"given": "Osiris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Molecular and Structural Physiology, School of Biological Sciences, Universidad Autónoma de Nuevo León, San Nicolas de los Garza, Nuevo León 66455, Mexico"
}
],
"family": "Rodriguez‑Sanchez",
"given": "Iram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Molecular Medicine Laboratory, Academic Unit of Human Medicine and Health Sciences, Autonomous University of Zacatecas, Zacatecas 98160, Mexico"
}
],
"family": "Martinez‑Fierro",
"given": "Margarita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Rojas‑Larios",
"given": "Fabian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Walle‑Guillen",
"given": "Mireya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Cardenas‑Aguilar",
"given": "Citlaly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Beas‑Guzman",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Chaviano‑Conesa",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, General Hospital of Zone No. 1 IMSS, Villa de Alvarez, Colima 28984, Mexico"
}
],
"family": "Garcia‑Garcia",
"given": "Hossana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular Medicine, School of Medicine, University of Colima, Colima 28040, Mexico"
}
],
"family": "Delgado‑Enciso",
"given": "Ivan",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Medicine",
"container-title-short": "Int J Mol Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T13:56:06Z",
"timestamp": 1644414966000
},
"deposited": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T10:00:21Z",
"timestamp": 1646215221000
},
"indexed": {
"date-parts": [
[
2024,
8,
19
]
],
"date-time": "2024-08-19T17:06:06Z",
"timestamp": 1724087166890
},
"is-referenced-by-count": 11,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
1,
14
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
1,
14
]
]
}
},
"member": "2249",
"original-title": [],
"prefix": "10.3892",
"published": {
"date-parts": [
[
2022,
1,
14
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
14
]
]
},
"publisher": "Spandidos Publications",
"reference": [
{
"DOI": "10.3389/fpubh.2020.00337",
"article-title": "The coronavirus disease (COVID-19) challenge in Mexico: A critical and forced reflection as individuals and society",
"author": "Caldera-Villalobos",
"doi-asserted-by": "publisher",
"first-page": "337",
"journal-title": "Front Public Health",
"key": "key20220302120007_b1-ijmm-0-0-05084",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0249090",
"article-title": "Magnitude of asymptomatic COVID-19 cases throughout the course of infection: A systematic review and meta-analysis",
"author": "Alene",
"doi-asserted-by": "publisher",
"first-page": "e0249090",
"journal-title": "PLoS One",
"key": "key20220302120007_b2-ijmm-0-0-05084",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72314 cases from the Chinese center for disease control and prevention",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA",
"key": "key20220302120007_b3-ijmm-0-0-05084",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.3892/etm.2021.10347",
"article-title": "Safety and efficacy of a COVID-19 treatment with nebulized and/or intravenous neutral electrolyzed saline combined with usual medical care vs. usual medical care alone: A randomized, open-label, controlled trial",
"author": "Delgado-Enciso",
"doi-asserted-by": "publisher",
"first-page": "915",
"journal-title": "Exp Ther Med",
"key": "key20220302120007_b4-ijmm-0-0-05084",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(21)00247-2",
"article-title": "COVID-19 and immune-mediated inflammatory diseases: Effect of disease and treatment on COVID-19 outcomes and vaccine responses",
"author": "Fagni",
"doi-asserted-by": "publisher",
"first-page": "e724",
"journal-title": "Lancet Rheumatol",
"key": "key20220302120007_b5-ijmm-0-0-05084",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/j.jcv.2020.104371",
"article-title": "Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "104371",
"journal-title": "J Clin Virol",
"key": "key20220302120007_b6-ijmm-0-0-05084",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6930e1",
"article-title": "Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network-United States, March-June 2020",
"author": "Tenforde",
"doi-asserted-by": "publisher",
"first-page": "993",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "key20220302120007_b7-ijmm-0-0-05084",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.2000413",
"article-title": "COVID-19 as an acute inflammatory disease",
"author": "Manjili",
"doi-asserted-by": "publisher",
"first-page": "12",
"journal-title": "J Immunol",
"key": "key20220302120007_b8-ijmm-0-0-05084",
"volume": "205",
"year": "2020"
},
{
"DOI": "10.3892/ijo.2020.5159",
"article-title": "Immune-checkpoint inhibitors from cancer to COVID-19: A promising avenue for the treatment of patients with COVID-19 (Review)",
"author": "Vivarelli",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "Int J Oncol",
"key": "key20220302120007_b9-ijmm-0-0-05084",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.3390/jcm9072084",
"article-title": "SARS-CoV-2: Repurposed drugs and novel therapeutic approaches-insights into chemical structure-biological activity and toxicological screening",
"author": "Dehelean",
"doi-asserted-by": "publisher",
"first-page": "2084",
"journal-title": "J Clin Med",
"key": "key20220302120007_b10-ijmm-0-0-05084",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3892/ijmm.2020.4608",
"article-title": "Comprehensive analysis of drugs to treat SARS-CoV-2 infection: Mechanistic insights into current COVID-19 therapies (Review)",
"author": "Nitulescu",
"doi-asserted-by": "publisher",
"first-page": "467",
"journal-title": "Int J Mol Med",
"key": "key20220302120007_b11-ijmm-0-0-05084",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.3892/ijmm.2020.4636",
"article-title": "A dissection of SARS-CoV2 with clinical implications (Review)",
"author": "Stancioiu",
"doi-asserted-by": "publisher",
"first-page": "489",
"journal-title": "Int J Mol Med",
"key": "key20220302120007_b12-ijmm-0-0-05084",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/j.bja.2020.08.050",
"article-title": "COVID-19-related organ dysfunction and management strategies on the intensive care unit: A narrative review",
"author": "Sherren",
"doi-asserted-by": "publisher",
"first-page": "912",
"journal-title": "Br J Anaesth",
"key": "key20220302120007_b13-ijmm-0-0-05084",
"volume": "125",
"year": "2020"
},
{
"DOI": "10.1016/j.fct.2020.111769",
"article-title": "SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19",
"author": "Tsatsakis",
"doi-asserted-by": "publisher",
"first-page": "111769",
"journal-title": "Food Chem Toxicol",
"key": "key20220302120007_b14-ijmm-0-0-05084",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1007/s40264-021-01089-5",
"article-title": "NSAIDs and COVID-19: A systematic review and meta-analysis",
"author": "Moore",
"doi-asserted-by": "publisher",
"first-page": "929",
"journal-title": "Drug Saf",
"key": "key20220302120007_b15-ijmm-0-0-05084",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2020.110982",
"article-title": "Early use of non-steroidal anti-inflammatory drugs in COVID-19 might reverse pathogenesis, prevent complications and improve clinical outcomes",
"author": "Kelleni",
"doi-asserted-by": "publisher",
"first-page": "110982",
"journal-title": "Biomed Pharmacother",
"key": "key20220302120007_b16-ijmm-0-0-05084",
"volume": "133",
"year": "2021"
},
{
"article-title": "Mefenamic acid: The evolution of a versatile NSAID",
"author": "Tripathi",
"first-page": "115",
"journal-title": "Indian J Clin Pract",
"key": "key20220302120007_b17-ijmm-0-0-05084",
"volume": "32",
"year": "2021"
},
{
"article-title": "Mefenamic acid as steroid-sparing anti-inflammatory drug during viral phase of Covid-19: 5 case reports",
"author": "Aggarwal",
"first-page": "759",
"journal-title": "Indian J Clin Pract",
"key": "key20220302120007_b18-ijmm-0-0-05084",
"volume": "31",
"year": "2021"
},
{
"article-title": "Study the antiviral activity of some derivatives of tetracycline and non-steroid anti inflammatory drugs towards dengue virus",
"author": "Rothan",
"first-page": "681",
"journal-title": "Trop Biomed",
"key": "key20220302120007_b19-ijmm-0-0-05084",
"volume": "30",
"year": "2013"
},
{
"article-title": "Repurposing mefenamic acid in the management of covid-19",
"author": "Aggarwal",
"first-page": "16",
"journal-title": "J Indian Med Assoc",
"key": "key20220302120007_b20-ijmm-0-0-05084",
"volume": "119",
"year": "2021"
},
{
"DOI": "10.1016/j.ad.2019.05.009",
"article-title": "Using the common terminology criteria for adverse events (CTCAE-version 5.0) to evaluate the severity of adverse events of anticancer therapies",
"author": "Freites-Martinez",
"doi-asserted-by": "publisher",
"first-page": "90",
"journal-title": "Actas Dermosifiliogr (Engl Ed)",
"key": "key20220302120007_b21-ijmm-0-0-05084",
"volume": "112",
"year": "2021"
},
{
"DOI": "10.1007/s00405-020-06284-1",
"article-title": "Symptomatology of COVID-19 from the otorhinolaryngology perspective: A survey of 223 SARS-CoV-2 RNA-positive patients",
"author": "Salepci",
"doi-asserted-by": "publisher",
"first-page": "525",
"journal-title": "Eur Arch Otorhinolaryngol",
"key": "key20220302120007_b22-ijmm-0-0-05084",
"volume": "278",
"year": "2021"
},
{
"article-title": "Clinical Management of COVID-19: Interim guidance",
"author": "World Health Organization",
"key": "key20220302120007_b23-ijmm-0-0-05084",
"year": "2020"
},
{
"DOI": "10.7554/eLife.58728",
"article-title": "Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission",
"author": "Rivett",
"doi-asserted-by": "publisher",
"first-page": "e58728",
"journal-title": "Elife",
"key": "key20220302120007_b24-ijmm-0-0-05084",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3899/jrheum.080182",
"article-title": "RAPID3 (routine assessment of patient index data 3), a rheumatoid arthritis index without formal joint counts for routine care: Proposed severity categories compared to disease activity score and clinical disease activity index categories",
"author": "Pincus",
"doi-asserted-by": "publisher",
"first-page": "2136",
"journal-title": "J Rheumatol",
"key": "key20220302120007_b25-ijmm-0-0-05084",
"volume": "35",
"year": "2008"
},
{
"article-title": "Sample size calculator. ClinCalc",
"author": "Kane",
"key": "key20220302120007_b26-ijmm-0-0-05084"
},
{
"DOI": "10.1248/bpb.24.701",
"article-title": "Inhibitory effects of anti-inflammatory drugs on interleukin-6 bioactivity",
"author": "Kang",
"doi-asserted-by": "publisher",
"first-page": "701",
"journal-title": "Biol Pharm Bull",
"key": "key20220302120007_b27-ijmm-0-0-05084",
"volume": "24",
"year": "2001"
},
{
"DOI": "10.1016/j.cytogfr.2021.06.002",
"article-title": "The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies",
"author": "Zhao",
"doi-asserted-by": "publisher",
"first-page": "2",
"journal-title": "Cytokine Growth Factor Rev",
"key": "key20220302120007_b28-ijmm-0-0-05084",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1038/ncomms12504",
"article-title": "Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models",
"author": "Daniels",
"doi-asserted-by": "publisher",
"first-page": "12504",
"journal-title": "Nat Commun",
"key": "key20220302120007_b29-ijmm-0-0-05084",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1016/j.antiviral.2016.01.006",
"article-title": "Mefenamic acid in combination with ribavirin shows significant effects in reducing chikungunya virus infection in vitro and in vivo",
"author": "Rothan",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Antiviral Res",
"key": "key20220302120007_b30-ijmm-0-0-05084",
"volume": "127",
"year": "2016"
},
{
"DOI": "10.1159/000333558",
"article-title": "In vitro/in vivo correlation of fast release mephenamic acid microspheres in humans",
"author": "Etman",
"doi-asserted-by": "publisher",
"first-page": "223",
"journal-title": "Med Princ Pract",
"key": "key20220302120007_b31-ijmm-0-0-05084",
"volume": "21",
"year": "2012"
},
{
"DOI": "10.1016/0378-5173(84)90093-0",
"article-title": "Correlation between dissolution rate and bioavailability of different commercial mefenamic acid capsules",
"author": "Shinkuma",
"doi-asserted-by": "publisher",
"first-page": "187",
"journal-title": "Int J Pharm",
"key": "key20220302120007_b32-ijmm-0-0-05084",
"volume": "21",
"year": "1984"
},
{
"DOI": "10.12688/f1000research.26359.1",
"article-title": "Targeting SARS-CoV-2 RNA-dependent RNA polymerase: An in silico drug repurposing for COVID-19",
"author": "Baby",
"doi-asserted-by": "publisher",
"first-page": "1166",
"journal-title": "F1000Res",
"key": "key20220302120007_b33-ijmm-0-0-05084",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2021.103595",
"article-title": "The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model",
"author": "Abdelnabi",
"doi-asserted-by": "publisher",
"first-page": "103595",
"journal-title": "EBioMedicine",
"key": "key20220302120007_b34-ijmm-0-0-05084",
"volume": "72",
"year": "2021"
},
{
"article-title": "Decreased biochemical progression in patients with castration-resistant prostate cancer using a novel mefenamic acid anti-inflammatory therapy: A randomized controlled trial",
"author": "Guzman-Esquivel",
"first-page": "4151",
"journal-title": "Oncol Lett",
"key": "key20220302120007_b35-ijmm-0-0-05084",
"volume": "19",
"year": "2020"
},
{
"article-title": "Improve cognitive impairment using mefenamic acid non-steroidal anti-inflammatory therapy: Additional beneficial effect found in a controlled clinical trial for prostate cancer therapy",
"author": "Melnikov",
"first-page": "4535",
"journal-title": "Am J Transl Res",
"key": "key20220302120007_b36-ijmm-0-0-05084",
"volume": "13",
"year": "2021"
},
{
"article-title": "Celecoxib versus mefenamic acid in the treatment of primary dysmenorrhea",
"author": "Basri",
"journal-title": "Horm Mol Biol Clin Investig",
"key": "key20220302120007_b37-ijmm-0-0-05084",
"volume": "41",
"year": "2020"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.spandidos-publications.com/10.3892/ijmm.2022.5084"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of the use of mefenamic acid combined with standard medical care vs. standard medical care alone for the treatment of COVID‑19: A randomized double‑blind placebo‑controlled trial",
"type": "journal-article",
"volume": "49"
}