Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study
et al., Lancet Rheumatology, doi:10.1016/S2665-9913(20)30305-2, Sep 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective patients with rheumatologic conditions showing zero of 10,703 COVID-19 deaths for HCQ patients versus 7 of 21,406 propensity matched control patients (not statistically significant). The average age of HCQ patients is slightly lower 64.8 versus 65.4 control.
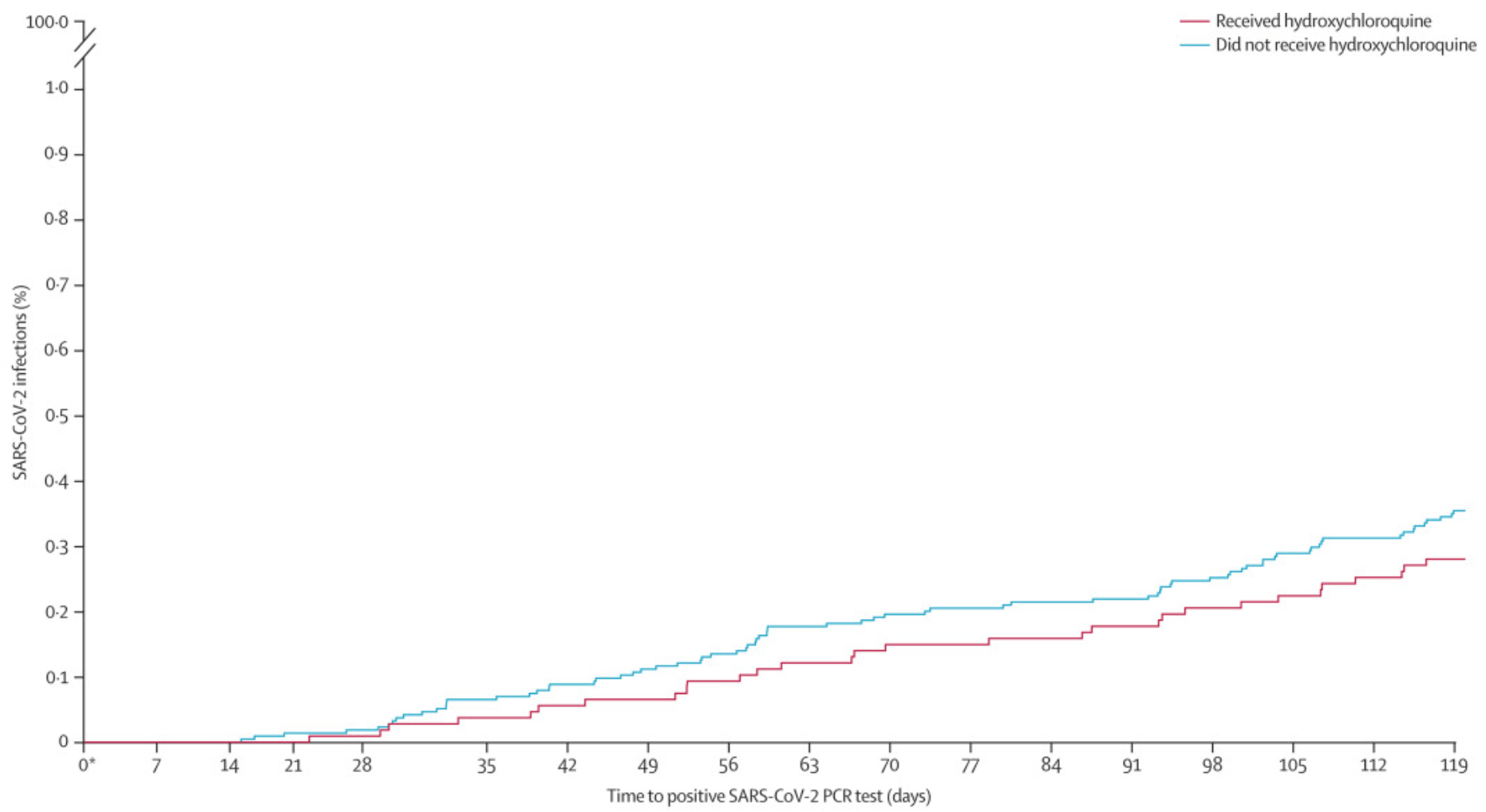
COVID-19 cases OR 0.79, p=0.27. There are several significant differences in the propensity matched patients that could affect results, e.g., 20.9% SLE versus 24.7%.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 91.3% lower, RR 0.09, p = 0.10, treatment 0 of 10,703 (0.0%), control 7 of 21,406 (0.0%), NNT 3058, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), COVID-19 mortality within all patients.
|
|
risk of death, 90.7% lower, RR 0.09, p = 0.19, treatment 0 of 31 (0.0%), control 7 of 78 (9.0%), NNT 11, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), mortality for infected patients.
|
|
risk of case, 20.9% lower, RR 0.79, p = 0.27, treatment 31 of 10,703 (0.3%), control 78 of 21,406 (0.4%), NNT 1338, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gentry et al., 21 Sep 2020, retrospective, database analysis, USA, peer-reviewed, 6 authors.
Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study
The Lancet Rheumatology, doi:10.1016/s2665-9913(20)30305-2
Background Hydroxychloroquine is one of several agents being evaluated in the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We aimed to examine whether patients with rheumatological conditions receiving chronic hydroxychloroquine therapy are at less risk of developing SARS-CoV-2 infection than those not receiving hydroxychloroquine.
Methods This retrospective cohort study included de-identified information of all veterans in the US Veterans Health Administration clinical administrative database aged 18 years or older with rheumatoid arthritis, systemic lupus erythematosus, or associated rheumatological conditions (based on International Classification of Diseases, 10th edition, diagnostic codes) who were alive on March 1, 2020. A propensity score was calculated for each patient, and each patient who was receiving hydroxychloroquine was matched to two patients who were not receiving hydroxychloroquine (controls). The primary endpoint was the proportion of patients with PCR-confirmed SARS-CoV-2 infection among those receiving chronic hydroxychloroquine versus the propensity-matched patients not receiving chronic hydroxychloroquine between March 1 and June 30, 2020. Secondary outcomes were hospital admission associated with SARS-CoV-2 infection; intensive care requirement associated with SARS-CoV-2 infection; mortality associated with SARS-CoV-2 infection; and overall rates of any hospital admission and mortality (ie, all cause). Multivariate logistic regression analysis was done to determine independent variables for the development of active SARS-CoV-2 infection. Findings Between March 1 and June 30, 2020, 10 703 patients receiving hydroxychloroquine and 21 406 patients not receiving hydroxychloroquine were included in the primary analysis. The incidence of active SARS-CoV-2 infections during the study period did not differ between patients receiving hydroxychloroquine and patients not receiving hydroxychloroquine (31 [0•3%] of 10 703 vs 78 [0•4%] of 21 406; odds ratio 0•79, 95% CI 0•52-1•20, p=0•27). There were no significant differences in secondary outcomes between the two groups in patients who developed active SARS-CoV-2 infection. For all patients in the study, overall mortality was lower in the hydroxychloroquine group than in the group of patients who did not receive hydroxychloroquine (odds ratio 0•70, 95% CI 0•55-0•89, p=0•0031). In multivariate logistic regression analysis, receipt of hydroxychloroquine was not associated with the development of active SARS-CoV-2 infection (odds ratio 0•79, 95% CI 0•51-1•42). Interpretation Hydroxychloroquine was not associated with a preventive effect against SARS-CoV-2 infection in a large group of patients with rheumatological conditions.
References
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19, N Engl J Med
Chu, Poon, Cheng, Initial viral load and the outcomes of SARS, CMAJ
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis
Gautret, Lagier, Parola, Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study, Travel Med Infect Dis
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents
Geleris, Sun, Platt, Observational study of hydroxychloroquine in hospitalized patients with Covid-19
Gendelman, Amital, Bragazzi, Watad, Chodick, Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis, Autoimmun Rev
Hernandez, Roman, Pasupuleti, Barboza, White, Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review, Ann Intern Med
Liu, Cao, Xu, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Mahévas, Tran, Roumier, Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data, BMJ
Paton, Goodall, Dunn, Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial, JAMA
Raebel, Schmittdiel, Karter, Konieczny, Steiner, Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases, Med Care
Rosenberg, Dufort, Udo, Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State, JAMA
Schrezenmeier, Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology, Nat Rev Rheumatol
Singh, Saag, Bridges, Jr, American College of Rheumatology guideline for the treatment of rheumatoid arthritis, Arthritis Rheumatol
Singh, Singh, Singh, Misra, Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis, Diabetes Metab Syndr
Sperber, Quraishi, Kalb, Panja, Stecher et al., Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells, J Rheumatol
Vitamin, Lai, Shih, Ko, Tang et al., 2565 (24•0%) nitrogen 1098 (10•3%) disease-modifying antirheumatic drug. *csDMARDs include hydroxychloroquine, methotrexate, lefunomide, and sulfasalazine; other csDMARD refers to agents other than hydroxychloroquine, Int J Antimicrob Agents
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Wang, Ye, Liu, Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence, Int J Antimicrob Agents
Who, Coronavirus disease (Covid-19) weekly epidemiologic update
Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin Infect Dis
Yazdany, Kim, Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know, Ann Intern Med
DOI record:
{
"DOI": "10.1016/s2665-9913(20)30305-2",
"ISSN": [
"2665-9913"
],
"URL": "http://dx.doi.org/10.1016/S2665-9913(20)30305-2",
"alternative-id": [
"S2665991320303052"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Rheumatology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2665-9913(20)30305-2"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2665-9913(20)30336-2"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Gentry",
"given": "Chris A",
"sequence": "first"
},
{
"affiliation": [],
"family": "Humphrey",
"given": "Mary Beth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thind",
"given": "Sharanjeet K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hendrickson",
"given": "Sage C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurdgelashvili",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Riley J",
"sequence": "additional",
"suffix": "II"
}
],
"container-title": "The Lancet Rheumatology",
"container-title-short": "The Lancet Rheumatology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
21
]
],
"date-time": "2020-09-21T22:31:52Z",
"timestamp": 1600727512000
},
"deposited": {
"date-parts": [
[
2021,
3,
20
]
],
"date-time": "2021-03-20T21:49:23Z",
"timestamp": 1616276963000
},
"indexed": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T08:26:06Z",
"timestamp": 1712132766548
},
"is-referenced-by-count": 35,
"issue": "11",
"issued": {
"date-parts": [
[
2020,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2020,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
1
]
],
"date-time": "2020-11-01T00:00:00Z",
"timestamp": 1604188800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2665991320303052?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2665991320303052?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e689-e697",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
11
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"article-title": "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges",
"author": "Lai",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/S2665-9913(20)30305-2_bib1",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105948",
"article-title": "Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/S2665-9913(20)30305-2_bib2",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"article-title": "An interactive web-based dashboard to track COVID-19 in real time",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "533",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S2665-9913(20)30305-2_bib4",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/S2665-9913(20)30305-2_bib5",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2665-9913(20)30305-2_bib6",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1038/s41584-020-0372-x",
"article-title": "Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology",
"author": "Schrezenmeier",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Nat Rev Rheumatol",
"key": "10.1016/S2665-9913(20)30305-2_bib7",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1002/art.39480",
"article-title": "2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Arthritis Rheumatol",
"key": "10.1016/S2665-9913(20)30305-2_bib8",
"volume": "68",
"year": "2016"
},
{
"DOI": "10.7326/M20-2496",
"article-title": "Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review",
"author": "Hernandez",
"doi-asserted-by": "crossref",
"first-page": "287",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2665-9913(20)30305-2_bib9",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with Covid-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"journal-title": "N Engl J Med",
"key": "10.1016/S2665-9913(20)30305-2_bib10",
"volume": "382",
"year": "2020"
},
{
"article-title": "Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data",
"author": "Mahévas",
"journal-title": "BMJ",
"key": "10.1016/S2665-9913(20)30305-2_bib11",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8630",
"article-title": "Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State",
"author": "Rosenberg",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/S2665-9913(20)30305-2_bib12",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2016638",
"article-title": "A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19",
"author": "Boulware",
"doi-asserted-by": "crossref",
"first-page": "517",
"journal-title": "N Engl J Med",
"key": "10.1016/S2665-9913(20)30305-2_bib14",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1097/MLR.0b013e31829b1d2a",
"article-title": "Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases",
"author": "Raebel",
"doi-asserted-by": "crossref",
"first-page": "S11",
"issue": "suppl 3",
"journal-title": "Med Care",
"key": "10.1016/S2665-9913(20)30305-2_bib16",
"volume": "51",
"year": "2013"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Cell Discov",
"key": "10.1016/S2665-9913(20)30305-2_bib18",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1503/cmaj.1040398",
"article-title": "Initial viral load and the outcomes of SARS",
"author": "Chu",
"doi-asserted-by": "crossref",
"first-page": "1349",
"journal-title": "CMAJ",
"key": "10.1016/S2665-9913(20)30305-2_bib19",
"volume": "171",
"year": "2004"
},
{
"DOI": "10.1001/jama.2012.6936",
"article-title": "Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial",
"author": "Paton",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "JAMA",
"key": "10.1016/S2665-9913(20)30305-2_bib20",
"volume": "308",
"year": "2012"
},
{
"article-title": "Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells",
"author": "Sperber",
"first-page": "803",
"journal-title": "J Rheumatol",
"key": "10.1016/S2665-9913(20)30305-2_bib21",
"volume": "20",
"year": "1993"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/S2665-9913(20)30305-2_bib22",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101663",
"article-title": "Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/S2665-9913(20)30305-2_bib23",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.7326/M20-1334",
"article-title": "Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know",
"author": "Yazdany",
"doi-asserted-by": "crossref",
"first-page": "754",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2665-9913(20)30305-2_bib24",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1016/j.dsx.2020.05.017",
"article-title": "Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "589",
"journal-title": "Diabetes Metab Syndr",
"key": "10.1016/S2665-9913(20)30305-2_bib25",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102566",
"article-title": "Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis",
"author": "Gendelman",
"doi-asserted-by": "crossref",
"journal-title": "Autoimmun Rev",
"key": "10.1016/S2665-9913(20)30305-2_bib26",
"volume": "19",
"year": "2020"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2665991320303052"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy",
"Rheumatology"
],
"subtitle": [],
"title": "Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "2"
}
