Estetrol Is Safe and Well Tolerated during Treatment of Hospitalized Men and Women with Moderate COVID-19 in a Randomized, Double-Blind Study
et al., Journal of Clinical Medicine, doi:10.3390/jcm12123928, NCT04801836, Jun 2023
RCT 175 hospitalized patients with moderate COVID-19 showing no significant benefit with estetrol (E4) treatment.
|
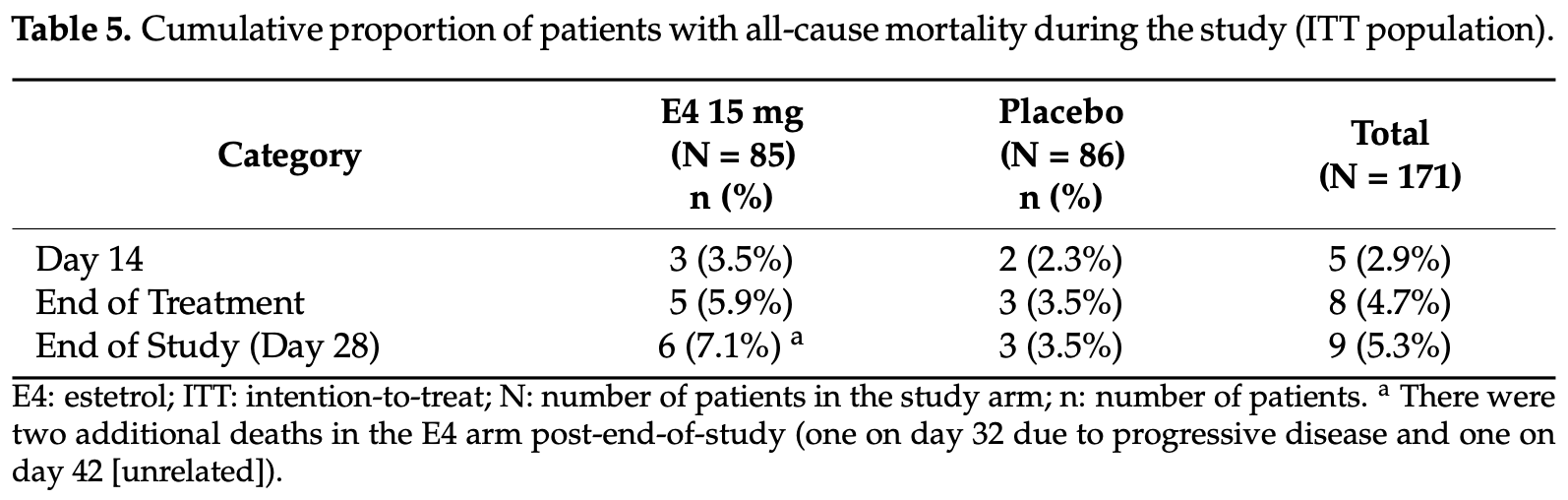
risk of death, 102.4% higher, RR 2.02, p = 0.33, treatment 6 of 85 (7.1%), control 3 of 86 (3.5%), day 28.
|
|
risk of no recovery, 116.8% higher, RR 2.17, p = 0.07, treatment 15 of 85 (17.6%), control 7 of 86 (8.1%), day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Foidart et al., 8 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, mean age 61.9, 7 authors, study period November 2020 - May 2021, trial NCT04801836 (history).
Contact: jfevaconsulting@gmail.com (corresponding author), gdixon@mithra.com, krzysimon@gmail.com, wulf@utianllc.com, fmauvais@tulane.edu, jonathan.douxfils@qualiblood.eu, phil.barrington@transcrip-group.com.
Estetrol Is Safe and Well Tolerated during Treatment of Hospitalized Men and Women with Moderate COVID-19 in a Randomized, Double-Blind Study
Journal of Clinical Medicine, doi:10.3390/jcm12123928
Epidemiological data suggest that the severe acute respiratory syndrome coronavirus 2 infection rate is higher in women than in men, but the death rate is lower, while women (>50 years) on menopausal hormone therapy (MHT) have a higher survival rate than those not on MHT. Classical oral estrogen enhances the synthesis of coagulation markers and may increase the risk of thromboembolic events that are common in coronavirus disease 2019 (COVID-19). The favorable hemostatic profile of estetrol (E4) might be suitable for use in women who are receiving estrogen treatment and contract COVID-19. A multicenter, randomized, double-blind, placebo-controlled, phase 2 study (NCT04801836) investigated the efficacy, safety, and tolerability of E4 versus placebo in hospitalized patients with moderate COVID-19. Eligible postmenopausal women and men (aged ≥ 18 years old) were randomized to E4 15 mg or placebo, once daily for 21 days, in addition to the standard of care (SoC). The primary efficacy endpoint of improvement in COVID-19 (percentage of patients recovered at day 28) between the placebo and E4 arms was not met. E4 was well tolerated, with no safety signals or thromboembolic events, suggesting that postmenopausal women can safely continue E4-based therapy in cases of moderate COVID-19 managed with SoC.
Conflicts of Interest: J.M.F. is co-founder of Mithra Pharmaceuticals, shareholder, and member of the board and chairman of the scientific committee. K.S. reports personal fees as a study investigator from Mithra Pharmaceuticals. W.H.U is a consultant and a member of the Scientific Advisory Board of Mithra Pharmaceuticals. F.M.J was a consultant to Mithra Pharmaceuticals. J.D. is the chief executive officer and founder of QUALIblood s.a. and reports personal fees from Daiichi-Sankyo, Diagnostica Stago, DOASense, Gedeon Richer, Mithra Pharmaceuticals, Norgine, Portola, Roche, Roche Diagnostics, Technoclone, and Werfen outside the submitted work. G.D. is an employee of Mithra Pharmaceuticals. P.B. was contracted by Mithra Pharmaceutical to design, monitor and report the study. Mithra Pharmaceuticals was involved in the design of the study; in the interpretation of data; in the review of the manuscript; and in the decision to publish the results.
References
Ader, Bouscambert-Duchamp, Hites, Peiffer-Smadja, Poissy et al., Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial, Lancet Infect. Dis, doi:10.1016/S1473-3099(21)00485-0
Al-Kuraishy, Hussien, Al-Naimi, Al-Buhadily, Al-Gareeb et al., Is ivermectin-Azithromycin combination the next step for COVID-19?, Biomed. Biotechnol. Res. J
Ali, Azher, Baqi, Binnie, Borgia et al., Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial, CMAJ, doi:10.1503/cmaj.211698
Aune, Oian, Omsjø, Osterud, Hormone replacement therapy reduces the reactivity of monocytes and platelets in whole blood-A beneficial effect on atherogenesis and thrombus formation?, Am. J. Obstet. Gynecol, doi:10.1016/0002-9378(95)90433-6
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of COVID-19-Final report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Clinicaltrials, Gov, Estradiol and Progesterone in Hospitalized COVID-19 Patients
Clinicaltrials, Gov, Estrogen Patch for COVID-19 Symptoms
Clinicaltrials, Gov, Estrogen Therapy in Non-Severe COVID-19 Patients
Clinicaltrials, Gov, Oestrogen Treatment for COVID-19 Symptoms
Costeira, Lee, Murray, Christiansen, Castillo-Fernandez et al., Estrogen and COVID-19 symptoms: Associations in women from the COVID Symptom Study, PLoS ONE, doi:10.1371/journal.pone.0257051
Dambha-Miller, Hinton, Joy, Feher, Lusignan, Mortality in COVID-19 amongst women on Hormone Replacement Therapy or Combined Oral Contraception: A cohort study, medRxiv, doi:10.1101/2021.02.16.21251853
Douxfils, Gaspard, Taziaux, Jost, Bouvy et al., Impact of estetrol (E4) on hemostasis, metabolism and bone turnover in postmenopausal women, Climacteric, doi:10.1080/13697137.2022.2139599
Douxfils, Klipping, Duijkers, Kinet, Mawet et al., Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters, Contraception, doi:10.1016/j.contraception.2020.08.015
Gencer, Lacy, Atzler, Van Der Vorst, Döring et al., Thrombohaemostatic, and Cardiovascular Mechanisms in COVID-19, Thromb. Haemost, doi:10.1055/s-0040-1718735
Goldman, Lye, Hui, Marks, Bruno et al., Remdesivir for 5 or 10 Days in Patients with Severe COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2015301
Gérard, Arnal, Jost, Douxfils, Lenfant et al., Profile of estetrol, a promising native estrogen for oral contraception and the relief of climacteric symptoms of menopause, Expert Rev. Clin. Pharmacol, doi:10.1080/17512433.2022.2054413
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2031994
Khan, Ansar Ahmed, The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases, Front. Immunol, doi:10.3389/fimmu.2015.00635
Marconi, Ramanan, De Bono, Kartman, Krishnan et al., Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00331-3
Morimont, Jost, Gaspard, Foidart, Dogné et al., Low Thrombin Generation in Users of a Contraceptive Containing Estetrol and Drospirenone, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgac511
Rosas, Brau, Waters, Go, Hunter et al., Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2028700
Rosas, Diaz, Gottlieb, Lobo, Robinson et al., Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial, Intensive Care Med, doi:10.1007/s00134-021-06507-x
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in patients hospitalized with COVID-19 pneumonia, N. Engl. J. Med, doi:10.1056/NEJMoa2030340
Seeland, Coluzzi, Simmaco, Mura, Bourne et al., Evidence for treatment with estradiol for women with SARS-CoV-2 infection, BMC Med, doi:10.1186/s12916-020-01851-z
Spinner, Gottlieb, Criner, Arribas López, Cattelan et al., Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.16349
Stone, Frigault, Serling-Boyd, Fernandes, Harvey et al., Efficacy of Tocilizumab in Patients Hospitalized with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2028836
The, Group, Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis, Lancet, doi:10.1016/S0140-6736(22)01109-6
The, Group, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)00676-0
Valera, Noirrit-Esclassan, Dupuis, Fontaine, Lenfant et al., Effect of estetrol, a selective nuclear estrogen receptor modulator, in mouse models of arterial and venous thrombosis, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2018.06.010
Vegivinti, Evanson, Lyons, Akosman, Barrett et al., Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials, BMC Infect. Dis, doi:10.1186/s12879-022-07068-0
Yoshida, Chu, Zu, Fox, Mauvais-Jarvis, Effect of menopausal hormone therapy on COVID-19 severe outcomes in women-A population-based study of the US National COVID Cohort Collaborative (N3C) data, Maturitas, doi:10.1016/j.maturitas.2022.10.005
Zheng, Ma, Zhang, Xie, COVID-19 and the cardiovascular system, Nat. Rev. Cardiol, doi:10.1038/s41569-020-0360-5
DOI record:
{
"DOI": "10.3390/jcm12123928",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm12123928",
"abstract": "<jats:p>Epidemiological data suggest that the severe acute respiratory syndrome coronavirus 2 infection rate is higher in women than in men, but the death rate is lower, while women (>50 years) on menopausal hormone therapy (MHT) have a higher survival rate than those not on MHT. Classical oral estrogen enhances the synthesis of coagulation markers and may increase the risk of thromboembolic events that are common in coronavirus disease 2019 (COVID-19). The favorable hemostatic profile of estetrol (E4) might be suitable for use in women who are receiving estrogen treatment and contract COVID-19. A multicenter, randomized, double-blind, placebo-controlled, phase 2 study (NCT04801836) investigated the efficacy, safety, and tolerability of E4 versus placebo in hospitalized patients with moderate COVID-19. Eligible postmenopausal women and men (aged ≥ 18 years old) were randomized to E4 15 mg or placebo, once daily for 21 days, in addition to the standard of care (SoC). The primary efficacy endpoint of improvement in COVID-19 (percentage of patients recovered at day 28) between the placebo and E4 arms was not met. E4 was well tolerated, with no safety signals or thromboembolic events, suggesting that postmenopausal women can safely continue E4-based therapy in cases of moderate COVID-19 managed with SoC.</jats:p>",
"alternative-id": [
"jcm12123928"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-8285-3573",
"affiliation": [
{
"name": "Mithra Pharmaceuticals, 4000 Liège, Belgium"
},
{
"name": "Department of Obstetrics and Gynecology, University of Liège, 4000 Liège, Belgium"
}
],
"authenticated-orcid": false,
"family": "Foidart",
"given": "Jean Michel",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases and Hepatology, Wrocław Medical University, 51149 Wrocław, Poland"
}
],
"family": "Simon",
"given": "Krzysztof",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Reproductive Biology, Case Western Reserve Medical School, Cleveland, OH 44106, USA"
}
],
"family": "Utian",
"given": "Wulf H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrinology and Metabolism, Tulane University School of Medicine, New Orleans, LA 70112, USA"
}
],
"family": "Mauvais-Jarvis",
"given": "Franck",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7644-5298",
"affiliation": [
{
"name": "Department of Pharmacy, Namur Thrombosis and Hemostasis Center, Faculty of Medicine, University of Namur, 5000 Namur, Belgium"
},
{
"name": "QUALIblood s.a., 5000 Namur, Belgium"
}
],
"authenticated-orcid": false,
"family": "Douxfils",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mithra Pharmaceuticals, 4000 Liège, Belgium"
}
],
"family": "Dixon",
"given": "Graham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "tranScrip Ltd., Wokingham RG41 5TP, Berkshire, UK"
}
],
"family": "Barrington",
"given": "Philip",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
9
]
],
"date-time": "2023-06-09T06:03:18Z",
"timestamp": 1686290598000
},
"deposited": {
"date-parts": [
[
2025,
1,
6
]
],
"date-time": "2025-01-06T09:19:02Z",
"timestamp": 1736155142000
},
"funder": [
{
"DOI": "10.13039/100031579",
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/100031579",
"id-type": "DOI"
}
],
"name": "Mithra Pharmaceuticals"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T19:30:55Z",
"timestamp": 1740166255621,
"version": "3.37.3"
},
"is-referenced-by-count": 2,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
6,
8
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2023,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
8
]
],
"date-time": "2023-06-08T00:00:00Z",
"timestamp": 1686182400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/12/12/3928/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3928",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
6,
8
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
8
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.4103/bbrj.bbrj_109_20",
"article-title": "Is ivermectin–Azithromycin combination the next step for COVID-19?",
"author": "Hussien",
"doi-asserted-by": "crossref",
"first-page": "101",
"journal-title": "Biomed. Biotechnol. Res. J.",
"key": "ref_1",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1055/s-0040-1718735",
"article-title": "Immunoinflammatory, Thrombohaemostatic, and Cardiovascular Mechanisms in COVID-19",
"author": "Gencer",
"doi-asserted-by": "crossref",
"first-page": "1629",
"journal-title": "Thromb. Haemost.",
"key": "ref_2",
"volume": "120",
"year": "2020"
},
{
"DOI": "10.1038/s41569-020-0360-5",
"article-title": "COVID-19 and the cardiovascular system",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "Nat. Rev. Cardiol.",
"key": "ref_3",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2015.00635",
"article-title": "The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases",
"author": "Khan",
"doi-asserted-by": "crossref",
"first-page": "635",
"journal-title": "Front. Immunol.",
"key": "ref_4",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1016/0002-9378(95)90433-6",
"article-title": "Hormone replacement therapy reduces the reactivity of monocytes and platelets in whole blood—A beneficial effect on atherogenesis and thrombus formation?",
"author": "Aune",
"doi-asserted-by": "crossref",
"first-page": "1816",
"journal-title": "Am. J. Obstet. Gynecol.",
"key": "ref_5",
"volume": "173",
"year": "1995"
},
{
"DOI": "10.1080/13697137.2022.2139599",
"article-title": "Impact of estetrol (E4) on hemostasis, metabolism and bone turnover in postmenopausal women",
"author": "Douxfils",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Climacteric",
"key": "ref_6",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1016/j.contraception.2020.08.015",
"article-title": "Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters",
"author": "Douxfils",
"doi-asserted-by": "crossref",
"first-page": "396",
"journal-title": "Contraception",
"key": "ref_7",
"volume": "102",
"year": "2020"
},
{
"DOI": "10.1080/17512433.2022.2054413",
"article-title": "Profile of estetrol, a promising native estrogen for oral contraception and the relief of climacteric symptoms of menopause",
"author": "Arnal",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "Expert Rev. Clin. Pharmacol.",
"key": "ref_8",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1210/clinem/dgac511",
"article-title": "Low Thrombin Generation in Users of a Contraceptive Containing Estetrol and Drospirenone",
"author": "Morimont",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "J. Clin. Endocrinol. Metab.",
"key": "ref_9",
"volume": "108",
"year": "2022"
},
{
"DOI": "10.1016/j.mce.2018.06.010",
"article-title": "Effect of estetrol, a selective nuclear estrogen receptor modulator, in mouse models of arterial and venous thrombosis",
"author": "Valera",
"doi-asserted-by": "crossref",
"first-page": "132",
"journal-title": "Mol. Cell. Endocrinol.",
"key": "ref_10",
"volume": "477",
"year": "2018"
},
{
"DOI": "10.1186/s12916-020-01851-z",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Seeland, U., Coluzzi, F., Simmaco, M., Mura, C., Bourne, P.E., Heiland, M., Preissner, R., and Preissner, S. (2020). Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med., 18."
},
{
"DOI": "10.1371/journal.pone.0257051",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Costeira, R., Lee, K.A., Murray, B., Christiansen, C., Castillo-Fernandez, J., Ni Lochlainn, M., Capdevila Pujol, J., Macfarlane, H., Kenny, L.C., and Buchan, I. (2021). Estrogen and COVID-19 symptoms: Associations in women from the COVID Symptom Study. PLoS ONE, 16."
},
{
"DOI": "10.1016/S1473-3099(20)30637-X",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "WHO Working Group on the Clinical Characterisation and Management of Covid Infection (2020). A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis., 20, e192–e197. Correction in Lancet Infect. Dis. 2020, 20, e250."
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—Final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "ref_14",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1101/2021.02.11.21249258",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "The RECOVERY Collaborative Group (2021). Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet, 397, 1637–1645."
},
{
"DOI": "10.1056/NEJMoa2028700",
"article-title": "Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1503",
"journal-title": "N. Engl. J. Med.",
"key": "ref_16",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1186/s12879-022-07068-0",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Vegivinti, C.T.R., Evanson, K.W., Lyons, H., Akosman, I., Barrett, A., Hardy, N., Kane, B., Keesari, P.R., Pulakurthi, Y.S., and Sheffels, E. (2022). Efficacy of antiviral therapies for COVID-19: A systematic review of randomized controlled trials. BMC Infect. Dis., 22."
},
{
"DOI": "10.1101/2021.02.16.21251853",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Dambha-Miller, H., Hinton, W., Joy, M., Feher, M., and Lusignan, S.d. (2021). Mortality in COVID-19 amongst women on Hormone Replacement Therapy or Combined Oral Contraception: A cohort study. medRxiv."
},
{
"DOI": "10.1016/j.maturitas.2022.10.005",
"article-title": "Effect of menopausal hormone therapy on COVID-19 severe outcomes in women—A population-based study of the US National COVID Cohort Collaborative (N3C) data",
"author": "Yoshida",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Maturitas",
"key": "ref_19",
"volume": "170",
"year": "2023"
},
{
"DOI": "10.1503/cmaj.211698",
"article-title": "Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "E242",
"journal-title": "CMAJ",
"key": "ref_20",
"volume": "194",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_21",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "WHO Solidarity Trial Consortium (2022). Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet, 399, 1941–1953."
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial",
"author": "Spinner",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "JAMA",
"key": "ref_23",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 Days in Patients with Severe COVID-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N. Engl. J. Med.",
"key": "ref_24",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "The RECOVERY Collaborative Group (2021). Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med., 384, 693–704."
},
{
"DOI": "10.1101/2022.03.02.22271623",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "The RECOVERY Collaborative Group (2022). Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet, 400, 359–368."
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir. Med.",
"key": "ref_27",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "N. Engl. J. Med.",
"key": "ref_28",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2030340",
"article-title": "Tocilizumab in patients hospitalized with COVID-19 pneumonia",
"author": "Salama",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "N. Engl. J. Med.",
"key": "ref_29",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2028836",
"article-title": "Efficacy of Tocilizumab in Patients Hospitalized with COVID-19",
"author": "Stone",
"doi-asserted-by": "crossref",
"first-page": "2333",
"journal-title": "N. Engl. J. Med.",
"key": "ref_30",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1007/s00134-021-06507-x",
"article-title": "Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1258",
"journal-title": "Intensive Care Med.",
"key": "ref_31",
"volume": "47",
"year": "2021"
},
{
"key": "ref_32",
"unstructured": "ClinicalTrials.gov (2023, May 19). Estrogen Patch for COVID-19 Symptoms, Available online: https://clinicaltrials.gov/ct2/show/NCT04359329."
},
{
"key": "ref_33",
"unstructured": "ClinicalTrials.gov (2023, May 19). Estrogen Therapy in Non-Severe COVID-19 Patients, Available online: https://clinicaltrials.gov/ct2/show/NCT04539626."
},
{
"key": "ref_34",
"unstructured": "ClinicalTrials.gov (2023, May 19). Oestrogen Treatment for COVID-19 Symptoms, Available online: https://clinicaltrials.gov/ct2/show/NCT04853069."
},
{
"key": "ref_35",
"unstructured": "ClinicalTrials.gov (2023, May 19). Estradiol and Progesterone in Hospitalized COVID-19 Patients, Available online: https://clinicaltrials.gov/ct2/show/NCT04865029."
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/12/12/3928"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Estetrol Is Safe and Well Tolerated during Treatment of Hospitalized Men and Women with Moderate COVID-19 in a Randomized, Double-Blind Study",
"type": "journal-article",
"volume": "12"
}