Bile acids and bile acid activated receptors in the treatment of Covid-19
et al., Biochemical Pharmacology, doi:10.1016/j.bcp.2023.115983, Dec 2023
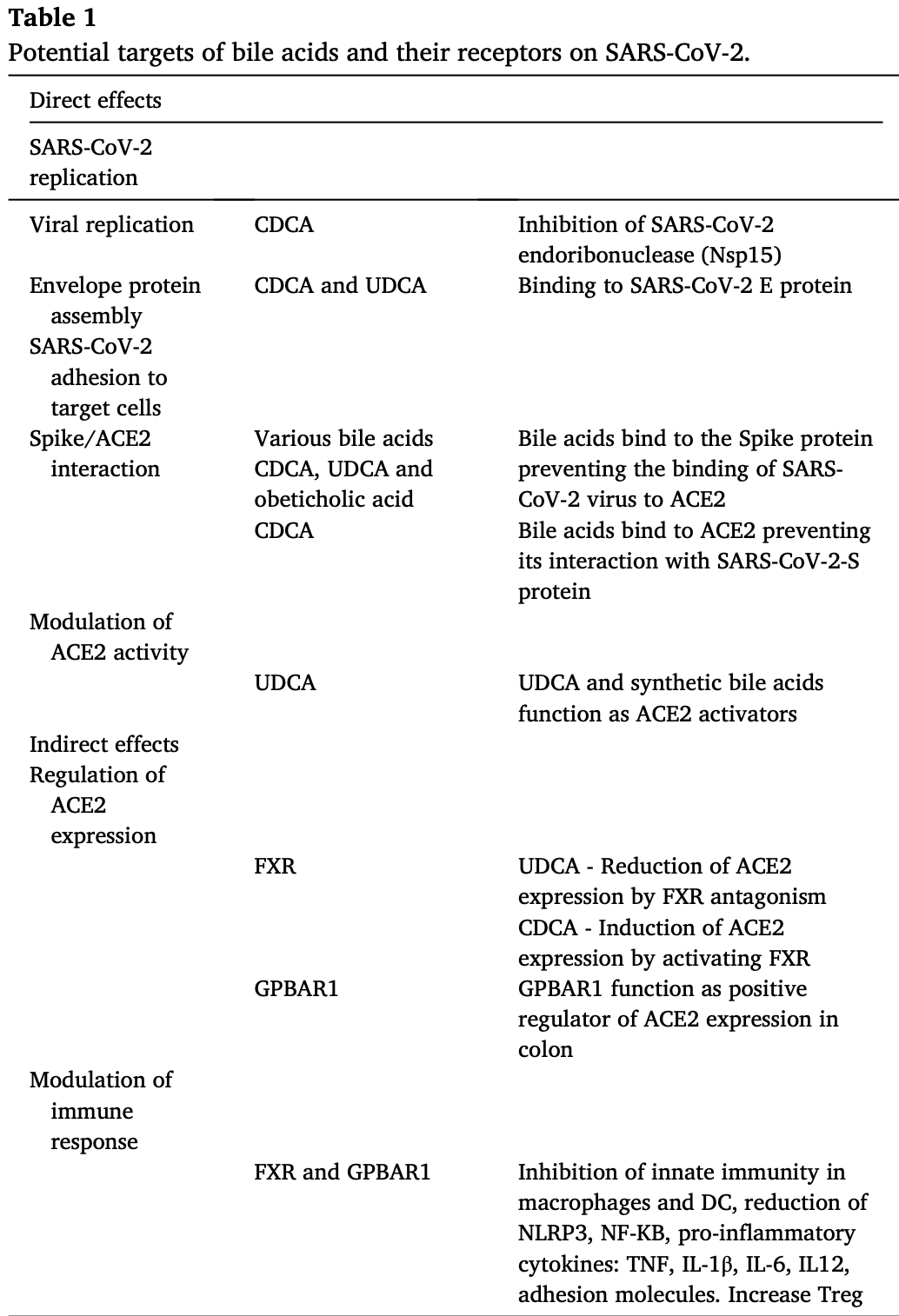
Review of the potential role of bile acids and bile acid activated receptors in modulating SARS-CoV-2 infectivity and inflammation in COVID-19. Authors discuss mechanisms by which bile acids like ursodeoxycholic acid (UDCA), chenodeoxycholic acid (CDCA), and obeticholic acid may inhibit binding of the SARS-CoV-2 spike protein to the ACE2 receptor, preventing viral entry into host cells. Additionally, bile acid receptors FXR and GPBAR1 regulate expression of ACE2, impacting susceptibility to infection, while also modulating inflammation through effects on immune pathways.
See Huang et al. for another review covering ursodeoxycholic acid for COVID-19.
Fiorucci et al., 9 Dec 2023, peer-reviewed, 6 authors.
Contact: stefano.fiorucci@unipg.it.
Bile acids and bile acid activated receptors in the treatment of Covid-19
Biochemical Pharmacology, doi:10.1016/j.bcp.2023.115983
Since its first outbreak in 2020, the pandemic caused by the Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) has caused the death of almost 7 million people worldwide. Vaccines have been fundamental in disease prevention and to reduce disease severity especially in patients with comorbidities. Nevertheless, treatment of COVID-19 has been proven difficult and several approaches have failed to prevent disease onset or disease progression, particularly in patients with comorbidities. Interrogation of drug data bases has been widely used since the beginning of pandemic to repurpose existing drugs/natural substances for the prevention/treatment of COVID-19. Steroids, including bile acids such as ursodeoxycholic acid (UDCA) and chenodeoxycholic acid (CDCA) have shown to be promising for their potential in modulating SARS-CoV-2/host interaction. Bile acids have proven to be effective in preventing binding of spike protein with the Angiotensin Converting Enzyme II (ACE2), thus preventing virus uptake by the host cells and inhibiting its replication, as well as in indirectly modulating immune response. Additionally, the two main bile acid activated receptors, GPBAR1 and FXR, have proven effective in modulating the expression of ACE2, suggesting an indirect role for these receptors in regulating SARS-CoV-2 infectiveness and immune response. In this review we have examined how the potential of bile acids and their receptors as anti-COVID-19 therapies and how these biochemical mechanisms translate into clinical efficacy.
SARS-CoV-2
SARS-CoV-2: An overview As reported by the WHO Coronavirus Dashboard (https://COVID-19. who.int), since its first outbreak in early 2020, the Coronavirus Disease 2019 (COVID-19) has resulted in the death of almost 7 million people worldwide [1] . The Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2), the disease-causing pathogen, still wanders among the worldwide population. However, immediately implemented and still ongoing vaccination campaigns against SARS-CoV-2 have granted the transition from the initial pandemic to the current endemic phase [2] . Vaccines have been fundamental in disease prevention, and it is estimated that nearly 80 % of the world population is fully vaccinated to
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Abdulrab, Al-Maweri, Halboub, Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm, Med. Hypotheses, doi:10.1016/j.mehy.2020.109897
Aguiar, Gomes De Lemos, Braz-Filho, Marques Da Fonseca, Silva Marinho et al., Synthesis and in silico study of chenodeoxycholic acid and its analogues as an alternative inhibitor of spike glycoprotein of SARS-CoV-2, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2022.2133010
Arendse, Danser, Poglitsch, Touyz, Burnett et al., Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure, Pharmacol. Rev, doi:10.1124/pr.118.017129
Babalghith, Al-Kuraishy, Al-Gareeb, De Waard, Al-Hamash et al., The role of berberine in Covid-19: potential adjunct therapy, Inflammopharmacology, doi:10.1007/s10787-022-01080-1
Bai, Zhong, Gao, Overview of SARS-CoV-2 genome-encoded proteins, Sci. China. Life Sci, doi:10.1007/s11427-021-1964-4
Benigni, Cassis, Remuzzi, Angiotensin II revisited: new roles in inflammation, immunology and aging, EMBO Mol. Med, doi:10.1002/emmm.201000080
Berberine, None, Altern. Med. Rev
Beyerstedt, Casaro, Rangel, COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/s10096-020-04138-6
Biagioli, Fiorucci, Bile acid activated receptors: Integrating immune and metabolic regulation in non-alcoholic fatty liver disease, Liver Res, doi:10.1016/J.LIVRES.2021.08.003
Biagioli, Marchianò, Carino, Di Giorgio, Santucci et al., Bile acids activated receptors in inflammatory bowel disease, Cells, doi:10.3390/cells10061281
Biagioli, Marchianò, Roselli, Di Giorgio, Bellini et al., GLP-1 Mediates Regulation of Colonic ACE2 Expression by the Bile Acid Receptor GPBAR1 in Inflammation, Cells, doi:10.3390/cells11071187
Biancolella, Colona, Mehrian-Shai, Watt, Luzzatto et al., COVID-19 2022 update: transition of the pandemic to the endemic phase, Hum. Genom, doi:10.1186/s40246-022-00392-1
Bourgonje, Abdulle, Timens, Hillebrands, Navis et al., Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19), J. Pathol, doi:10.1002/path.5471
Boyer, Bile formation and secretion, Compr. Physiol, doi:10.1002/cphy.c120027
Brevini, Maes, Webb, John, Fuchs et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Canales, Pérez-Campos Mayoral, Hernández-Huerta, Sánchez Navarro, Matias-Cervantes et al., Interaction of Spike protein and lipid membrane of SARS-CoV-2 with Ursodeoxycholic acid, an in-silico analysis, Sci. Rep, doi:10.1038/s41598-021-01705-5
Cao, Hu, Li, Wang, Xu et al., Anti-SARS-CoV-2 Potential of Artemisinins In Vitro, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00522
Carino, Biagioli, Marchianò, Fiorucci, Zampella et al., Ursodeoxycholic acid is a GPBAR1 agonist and resets liver/intestinal FXR signaling in a model of diet-induced dysbiosis and NASH, Biochim. Biophys. Acta Mol. Cell. Biol. Lipids, doi:10.1016/j.bbalip.2019.07.006
Carino, Marchianò, Biagioli, Bucci, Vellecco et al., Agonism for the bile acid receptor GPBAR1 reverses liver and vascular damage in a mouse model of steatohepatitis, FASEB J, doi:10.1096/fj.201801373RR
Carino, Moraca, Fiorillo, Marchianò, Sepe et al., Hijacking SARS-CoV-2/ACE2 receptor interaction by natural and semi-synthetic steroidal agents acting on functional pockets on the receptor binding domain, BioRxiv, doi:10.1101/2020.06.10.144964
Chan, Kok, Zhu, Chu, To et al., Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan, Emerg. Microb. Infect, doi:10.1080/22221751.2020.1719902
Chen, Cassaro, Physiology, Bile Acids
Chiang, Bile acids: regulation of synthesis: thematic review series: bile acids, J. Lipid Res, doi:10.1194/jlr.R900010-JLR200
Choi, Shin, Kang, Park, Beck, Target-Centered Drug Repurposing Predictions of Human Angiotensin-Converting Enzyme 2 (ACE2) and Transmembrane Protease Serine Subtype 2 (TMPRSS2) Interacting Approved Drugs for Coronavirus Disease 2019 (COVID-19) Treatment through a Drug-Target Interact, Viruses, doi:10.3390/v12111325
Chourasia, Koppula, Battu, Ouseph, Singh, EGCG, a Green Tea Catechin, as a Potential Therapeutic Agent for Symptomatic and Asymptomatic SARS-CoV-2 Infection, Molecules, doi:10.3390/molecules26051200
Colapietro, Angelotti, Masetti, Shiffer, Pugliese et al., Ursodeoxycholic Acid does not improve COVID-19 outcome in hospitalized patients, Viruses, doi:10.3390/v15081738
Comar, Otter, Pfannenstiel, Doerger, Renner et al., MERS-CoV endoribonuclease and accessory proteins jointly evade host innate immunity during infection of lung and nasal epithelial cells, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.2123208119
Copple, Li, Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules, Pharmacol. Res, doi:10.1016/j.phrs.2015.12.007
Delgado-Roche, Mesta, Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection, Arch. Med. Res, doi:10.1016/j.arcmed.2020.04.019
Deng, Baker, An "Old" protein with a new story: Coronavirus endoribonuclease is important for evading host antiviral defenses, Virology, doi:10.1016/j.virol.2017.12.024
Deng, Yin, Chen, Zeng, Clinical determinants for fatality of 44,672 patients with COVID-19, Crit. Care, doi:10.1186/s13054-020-02902-w
Dhama, Nainu, Frediansyah, Yatoo, Mohapatra et al., Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies, J. Infect Public Health, doi:10.1016/j.jiph.2022.11.024
Durairajan, Singh, Saravanan, Namachivayam, Radhakrishnan et al., Gastrointestinal manifestations of SARS-CoV-2: transmission, pathogenesis, immunomodulation, microflora dysbiosis, and clinical implications, Viruses, doi:10.3390/v15061231
Festa, Renga, D'amore, Sepe, Finamore et al., Exploitation of cholane scaffold for the discovery of potent and selective farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (GP-BAR1) ligands, J. Med. Chem, doi:10.1021/jm501273r
Fiorillo, Marchianò, Moraca, Sepe, Carino et al., Discovery of Bile Acid Derivatives as Potent ACE2 Activators by Virtual Screening and Essential Dynamics, J. Chem. Inf. Model, doi:10.1021/acs.jcim.1c01126
Fiorucci, Carino, Baldoni, Santucci, Costanzi et al., Bile Acid Signaling in Inflammatory Bowel Diseases, Dig. Dis. Sci, doi:10.1007/s10620-020-06715-3
Fiorucci, Distrutti, Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders, Trends Mol. Med, doi:10.1016/j.molmed.2015.09.001
Fiorucci, Distrutti, Carino, Zampella, Biagioli, Bile acids and their receptors in metabolic disorders, Prog. Lipid. Res, doi:10.1016/j.plipres.2021.101094
Fiorucci, Distrutti, The pharmacology of bile acids and their receptors, Handb. Exp. Pharmacol, doi:10.1007/164_2019_238
Fiorucci, Urbani, Distrutti, Bile Acids and SARS-CoV-2: Ursodeoxycholic Acid as a Potential Treatment of COVID-19, Recent Adv. Inflamm. Allergy Drug Discov, doi:10.2174/2772270817666230601124326
Fiorucci, Urbani, Role of mRAGEs and ACE2 in SARS-CoV-2-Related Inflammation, Recent Adv. Inflamm. Allergy Drug Discov, doi:10.2174/277227081601221018140453
Fiorucci, Zampella, Ricci, Distrutti, Biagioli, Immunomodulatory functions of FXR, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2022.111650
Geng, Lin, Bacterial bile salt hydrolase: an intestinal microbiome target for enhanced animal health, Anim Heal. Res Rev, doi:10.1017/S1466252316000153
Ghosh, Chakraborty, Biswas, Chowdhuri, Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors -an in silico docking and molecular dynamics simulation study, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1779818
Ghosh, Nandi, Saha, A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein, Int. Immunopharmacol, doi:10.1016/j.intimp.2022.108565
Guo, Peng, Hao, Ji, Zhang et al., Dihydroartemisinin promoted FXR expression independent of YAP1 in hepatocellular carcinoma, FASEB J
Hang, Paik, Yao, Kim, Trinath et al., Bile acid metabolites control T(H)17 and T (reg) cell differentiation, Nature, doi:10.1038/s41586-019-1785-z
Hassine, Covid-19 vaccines and variants of concern: A review, Rev. Med. Virol
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00418-x
John, Bastaich, Webb, Brevini, Moon et al., Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis, J. Intern. Med, doi:10.1111/joim.13630
Kciuk, Mujwar, Rani, Munjal, Gielecińska et al., Computational bioprospecting guggulsterone against ADP ribose phosphatase of SARS-CoV-2, Molecules, doi:10.3390/molecules27238287
Ko, Lee, Kim, Jo, Kumar et al., Antiinflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages, PLoS One
Kowalczuk, Bröer, Tietze, Vanslambrouck, Rasko et al., A protein complex in the brush-border membrane explains a Hartnup disorder allele, FASEB J, doi:10.1096/fj.08-107300
Kremsner, Krishna, Antimalarial combinations, Lancet (Lond., Engl.), doi:10.1016/S0140-6736(04)16680-4
Lamers, Beumer, Van Der Vaart, Knoops, Puschhof et al., SARS-CoV-2 productively infects human gut enterocytes, Science, doi:10.1126/science.abc1669
Lapenna, Ciofani, Festi, Neri, Pierdomenico et al., Antioxidant properties of ursodeoxycholic acid, Biochem. Pharmacol, doi:10.1016/s0006-2952(02)01391-6
Lefebvre, Cariou, Lien, Kuipers, Staels, Role of bile acids and bile acid receptors in metabolic regulation, Physiol. Rev, doi:10.1152/physrev.00010.2008
Li, Zhu, Cui, Lin, Li, Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2023.1178590
Lickteig, Csanaky, Pratt-Hyatt, Klaassen, Activation of Constitutive Androstane Receptor (CAR) in Mice Results in Maintained Biliary Excretion of Bile Acids Despite a Marked Decrease of Bile Acids in Liver, Toxicol. Sci, doi:10.1093/toxsci/kfw054
Lin, Zeng, Mai, Gao, Fang et al., Expression of ACE2, TMPRSS2, and SARS-CoV-2 nucleocapsid protein in gastrointestinal tissues from COVID-19 patients and association with gastrointestinal symptoms, Am. J. Med. Sci, doi:10.1016/j.amjms.2023.08.014
Liston, Whyte, Bile acids mediate signaling between microbiome and the immune system, Immunol. Cell Biol, doi:10.1111/imcb.12332
Liu, Bodnar, Meng, Khan, Wang et al., Epigallocatechin gallate from green tea effectively blocks infection of SARS-CoV-2 and new variants by inhibiting spike binding to ACE2 receptor, Cell Biosci, doi:10.1186/s13578-021-00680-8
Liu, Wang, Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children, Liver Int. Off. J. Int. Assoc. Study Liver, doi:10.1111/liv.15660
Ma, Hu, Wang, Choza, Wang, Drug-repurposing screening identified tropifexor as a SARS-CoV-2 papain-like protease inhibitor, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00629
Ma, Luo, Deng, Yang, Wang et al., Antibiotic-Induced Primary Biles Inhibit SARS-CoV-2 Endoribonuclease Nsp15 Activity in Mouse Gut, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.896504
Macierzanka, Torcello-Gómez, Jungnickel, Maldonado-Valderrama, Bile salts in digestion and transport of lipids, Adv. Colloid Interface Sci, doi:10.1016/j.cis.2019.102045
Makishima, Lu, Xie, Whitfield, Domoto et al., Vitamin D receptor as an intestinal bile acid sensor, Science, doi:10.1126/science.1070477
Makishima, Okamoto, Repa, Tu, Learned et al., Identification of a nuclear receptor for bile acids, Science, doi:10.1126/science.284.5418.1362
Marrone, Covino, Merra, Piccioni, Amodeo et al., Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts, Liver Int, Off. J. Int. Assoc. Study Liver, doi:10.1111/liv.15736
Martín Sánchez, Martínez-Sellés, Molero García, Moreno Guillén, Rodríguez-Artalejo et al., Insights for COVID-19 in 2023, Rev. Esp. Quimioter. Publ. Of. La Soc. Esp. Quimioter, doi:10.37201/req/122.2022
Maruyama, Miyamoto, Nakamura, Tamai, Okada et al., Identification of membrane-type receptor for bile acids (M-BAR), Biochem. Biophys. Res. Commun, doi:10.1016/s0006-291x(02)02550-0
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet (Lond., Engl.), doi:10.1016/S0140-6736(20)30628-0
Mencarelli, Renga, Palladino, Distrutti, Fiorucci, The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease, Biochem. Pharmacol, doi:10.1016/j.bcp.2009.06.026
Nagahashi, Yuza, Hirose, Nakajima, Ramanathan et al., The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases, J. Lipid Res, doi:10.1194/jlr.R069286
Nagle, Ferreira, Zhou, Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives, Phytochemistry, doi:10.1016/j.phytochem.2006.06.020
Narkhede, Pise, Cheke, Shinde, Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences, Nat. Products Bioprospect, doi:10.1007/s13659-020-00253-1
Ohishi, Hishiki, Baig, Rajpoot, Saqib et al., Epigallocatechin gallate (EGCG) attenuates severe acute respiratory coronavirus disease 2 (SARS-CoV-2) infection by blocking the interaction of SARS-CoV-2 spike protein receptor-binding domain to human angiotensin-converting enzyme 2, PLoS One
Parks, Blanchard, Bledsoe, Chandra, Consler et al., Bile acids: natural ligands for an orphan nuclear receptor, Science, doi:10.1126/science.284.5418.1365
Potdar, Dube, Naito, Li, Botwin et al., Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease, Gastroenterology, doi:10.1053/j.gastro.2020.10.041
Qiu, Liu, Mo, Liu, Chen et al., Immunoregulation by Artemisinin and Its Derivatives: A New Role for Old Antimalarial Drugs, Front. Immunol, doi:10.3389/fimmu.2021.751772
Renga, Migliorati, Mencarelli, Cipriani, D'amore et al., Farnesoid X receptor suppresses constitutive androstane receptor activity at the multidrug resistance protein-4 promoter, Biochim. Biophys. Acta, doi:10.1016/j.bbagrm.2011.01.008
Ridlon, Kang, Hylemon, Bile salt biotransformations by human intestinal bacteria, J. Lipid Res, doi:10.1194/jlr.R500013-JLR200
Rollins, Klaassen, Biliary excretion of drugs in man, Clin. Pharmacokinet, doi:10.2165/00003088-197904050-00003
Russell, The enzymes, regulation, and genetics of bile acid synthesis, Annu. Rev. Biochem, doi:10.1146/annurev.biochem.72.121801.161712
Sahin, Karikó, Türeci, mRNA-based therapeutics-developing a new class of drugs, Nat. Rev. Drug Discov, doi:10.1038/nrd4278
Shen, Yi, Sun, Bi, Du et al., Proteomic and Metabolomic Characterization of COVID-19 Patient Sera, Cell, doi:10.1016/j.cell.2020.05.032
Shu, Li, Cao, Li, Zhou et al., Berberine Alleviates Nonalcoholic Steatohepatitis Through Modulating Gut Microbiota Mediated Intestinal FXR Activation, Front. Pharmacol, doi:10.3389/fphar.2021.750826
Singer, Camargo, Ramadan, Schäfer, Mariotta et al., Defective intestinal amino acid absorption in Ace2 null mice, Am. J. Physiol. Gastrointest. Liver Physiol, doi:10.1152/ajpgi.00140.2012
Staels, Fonseca, Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration, Diab. Care, doi:10.2337/dc09-S355
Staudinger, Goodwin, Jones, Hawkins-Brown, Mackenzie et al., The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.051551698
Thuy, Bao, Moon, Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells, Biomed. Pharmacother, doi:10.1016/j.biopha.2022.113021
Tully, Rucker, Chianelli, Williams, Vidal et al., Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH), J. Med. Chem, doi:10.1021/acs.jmedchem.7b00907
Turner, ACE2 cell biology, regulation, and physiological functions, Prot. Arm Renin Angiotensin Syst, doi:10.1016/B978-0-12-801364-9.00025-0
Valdés, Moreno, Rello, Orduña, Bernardo et al., Metabolomics study of COVID-19 patients in four different clinical stages, Sci. Rep, doi:10.1038/s41598-022-05667-0
Vavassori, Mencarelli, Renga, Distrutti, Fiorucci, The bile acid receptor FXR is a modulator of intestinal innate immunity, J. Immunol, doi:10.4049/jimmunol.0803978
Verstockt, Verstockt, Abdu Rahiman, Ke, Arnauts et al., Intestinal Receptor of SARS-CoV-2 in Inflamed IBD Tissue Seems Downregulated by HNF4A in Ileum and Upregulated by Interferon Regulating Factors in Colon, J. Crohns Colitis, doi:10.1093/ecco-jcc/jjaa185
Wagner, Halilbasic, Marschall, Zollner, Fickert et al., CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice, Hepatology, doi:10.1002/hep.20784
Wang, Chen, Hollister, Sowers, Forman, Endogenous bile acids are ligands for the nuclear receptor FXR/BAR, Mol. Cell, doi:10.1016/s1097-2765(00)80348-2
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Wu, Zhao, Yu, Chen, Wang et al., A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Yadav, Choudhury, Kumar, Bhatia, Virtual repurposing of ursodeoxycholate and chenodeoxycholate as lead candidates against SARS-Cov2-Envelope protein: A molecular dynamics investigation, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1868339
Yamada, Sugimoto, Guggulsterone and its role in chronic diseases, Adv. Exp. Med. Biol, doi:10.1007/978-3-319-41342-6_15
Yeoh, Zuo, Lui, Zhang, Liu et al., Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Zuo, Zhang, Lui, Yeoh, Li et al., Alterations in gut microbiota of patients with COVID-19 during time of hospitalization, Gastroenterology, doi:10.1053/j.gastro.2020.05.048
DOI record:
{
"DOI": "10.1016/j.bcp.2023.115983",
"ISSN": [
"0006-2952"
],
"URL": "http://dx.doi.org/10.1016/j.bcp.2023.115983",
"alternative-id": [
"S0006295223005762"
],
"article-number": "115983",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Bile acids and bile acid activated receptors in the treatment of Covid-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Biochemical Pharmacology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.bcp.2023.115983"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier Inc."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3816-4222",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fiorucci",
"given": "Stefano",
"sequence": "first"
},
{
"affiliation": [],
"family": "Urbani",
"given": "Ginevra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biagioli",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sepe",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Distrutti",
"given": "Eleonora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zampella",
"given": "Angela",
"sequence": "additional"
}
],
"container-title": "Biochemical Pharmacology",
"container-title-short": "Biochemical Pharmacology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
9
]
],
"date-time": "2023-12-09T16:10:08Z",
"timestamp": 1702138208000
},
"deposited": {
"date-parts": [
[
2023,
12,
14
]
],
"date-time": "2023-12-14T05:20:04Z",
"timestamp": 1702531204000
},
"indexed": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T00:41:27Z",
"timestamp": 1702600887050
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0006295223005762?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0006295223005762?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "115983",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1186/s13054-020-02902-w",
"article-title": "Clinical determinants for fatality of 44,672 patients with COVID-19",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "179",
"journal-title": "Crit. Care",
"key": "10.1016/j.bcp.2023.115983_b0005",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1186/s40246-022-00392-1",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0010",
"unstructured": "M. Biancolella, V.L. Colona, R. Mehrian-Shai, J.L. Watt, L. Luzzatto, G. Novelli, J.K. V Reichardt, COVID-19 2022 update: transition of the pandemic to the endemic phase, Hum. Genom. 16 (2022) 19. https://doi.org/10.1186/s40246-022-00392-1."
},
{
"DOI": "10.1038/s41586-020-2008-3",
"article-title": "A new coronavirus associated with human respiratory disease in China",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "Nature",
"key": "10.1016/j.bcp.2023.115983_b0015",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1007/s11427-021-1964-4",
"article-title": "Overview of SARS-CoV-2 genome-encoded proteins",
"author": "Bai",
"doi-asserted-by": "crossref",
"first-page": "280",
"journal-title": "Sci. China. Life Sci.",
"key": "10.1016/j.bcp.2023.115983_b0020",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2020.1719902",
"article-title": "Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Emerg. Microb. Infect.",
"key": "10.1016/j.bcp.2023.115983_b0025",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2313",
"article-title": "Covid-19 vaccines and variants of concern: A review",
"author": "Hadj Hassine",
"doi-asserted-by": "crossref",
"first-page": "e2313",
"journal-title": "Rev. Med. Virol.",
"key": "10.1016/j.bcp.2023.115983_b0030",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1126/science.abb2507",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0035",
"unstructured": "D. Wrapp, N. Wang, K.S. Corbett, J.A. Goldsmith, C.L. Hsieh, O. Abiona, B.S. Graham, J.S. McLellan, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science (80-.). 367 (2020) 1260–1263. https://doi.org/10.1126/science.abb2507."
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 entry into cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "10.1016/j.bcp.2023.115983_b0040",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.intimp.2022.108565",
"article-title": "A review on evolution of emerging SARS-CoV-2 variants based on spike glycoprotein",
"author": "Ghosh",
"doi-asserted-by": "crossref",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.bcp.2023.115983_b0045",
"volume": "105",
"year": "2022"
},
{
"DOI": "10.1007/s10096-020-04138-6",
"article-title": "COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection",
"author": "Beyerstedt",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Eur. J. Clin. Microbiol. Infect. Dis.",
"key": "10.1016/j.bcp.2023.115983_b0050",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.bcp.2023.115983_b0055",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/B978-0-12-801364-9.00025-0",
"article-title": "ACE2 cell biology, regulation, and physiological functions",
"author": "Turner",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Prot. Arm Renin Angiotensin Syst.",
"key": "10.1016/j.bcp.2023.115983_b0060",
"year": "2015"
},
{
"DOI": "10.2174/277227081601221018140453",
"article-title": "Role of mRAGEs and ACE2 in SARS-CoV-2-Related Inflammation",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Recent Adv. Inflamm. Allergy Drug Discov.",
"key": "10.1016/j.bcp.2023.115983_b0065",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1152/ajpgi.00140.2012",
"article-title": "Defective intestinal amino acid absorption in Ace2 null mice",
"author": "Singer",
"doi-asserted-by": "crossref",
"first-page": "G686",
"journal-title": "Am. J. Physiol. Gastrointest. Liver Physiol.",
"key": "10.1016/j.bcp.2023.115983_b0070",
"volume": "303",
"year": "2012"
},
{
"DOI": "10.1096/fj.08-107300",
"article-title": "A protein complex in the brush-border membrane explains a Hartnup disorder allele",
"author": "Kowalczuk",
"doi-asserted-by": "crossref",
"first-page": "2880",
"journal-title": "FASEB J.",
"key": "10.1016/j.bcp.2023.115983_b0075",
"volume": "22",
"year": "2008"
},
{
"DOI": "10.1126/science.abc1669",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0080",
"unstructured": "M.M. Lamers, J. Beumer, J. van der Vaart, K. Knoops, J. Puschhof, T.I. Breugem, R.B.G. Ravelli, J. Paul van Schayck, A.Z. Mykytyn, H.Q. Duimel, E. van Donselaar, S. Riesebosch, H.J.H. Kuijpers, D. Schipper, W.J. van de Wetering, M. de Graaf, M. Koopmans, E. Cuppen, P.J. Peters, B.L. Haagmans, H. Clevers, SARS-CoV-2 productively infects human gut enterocytes, Science (80-.). 369 (2020) 50–54. https://doi.org/10.1126/science.abc1669."
},
{
"DOI": "10.1002/path.5471",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0085",
"unstructured": "A.R. Bourgonje, A.E. Abdulle, W. Timens, J.-L. Hillebrands, G.J. Navis, S.J. Gordijn, M.C. Bolling, G. Dijkstra, A.A. Voors, A.D.M.E. Osterhaus, P.H.J. van der Voort, D.J. Mulder, H. van Goor, Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19), J. Pathol. 251 (2020) 228–248. https://doi.org/https://doi.org/10.1002/path.5471."
},
{
"DOI": "10.1016/j.amjms.2023.08.014",
"article-title": "Expression of ACE2, TMPRSS2, and SARS-CoV-2 nucleocapsid protein in gastrointestinal tissues from COVID-19 patients and association with gastrointestinal symptoms",
"author": "Lin",
"doi-asserted-by": "crossref",
"journal-title": "Am. J. Med. Sci.",
"key": "10.1016/j.bcp.2023.115983_b0090",
"year": "2023"
},
{
"DOI": "10.1038/nrd4278",
"article-title": "mRNA-based therapeutics–developing a new class of drugs",
"author": "Sahin",
"doi-asserted-by": "crossref",
"first-page": "759",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.bcp.2023.115983_b0095",
"volume": "13",
"year": "2014"
},
{
"DOI": "10.1016/j.jiph.2022.11.024",
"article-title": "Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies",
"author": "Dhama",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "J. Infect Public Health.",
"key": "10.1016/j.bcp.2023.115983_b0100",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.37201/req/122.2022",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0105",
"unstructured": "F.J. Martín Sánchez, M. Martínez-Sellés, J.M. Molero García, S. Moreno Guillén, F.J. Rodríguez-Artalejo, J. Ruiz-Galiana, R. Cantón, P. De Lucas Ramos, A. García-Botella, A. García-Lledó, T. Hernández-Sampelayo, J. Gómez-Pavón, J. González Del Castillo, M.C. Martín-Delgado, E. Bouza, Insights for COVID-19 in 2023., Rev. Esp. Quimioter. Publ. Of. La Soc. Esp. Quimioter. 36 (2023) 114–124. https://doi.org/10.37201/req/122.2022."
},
{
"DOI": "10.2174/2772270817666230601124326",
"article-title": "Bile Acids and SARS-CoV-2: Ursodeoxycholic Acid as a Potential Treatment of COVID-19",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Recent Adv. Inflamm. Allergy Drug Discov.",
"key": "10.1016/j.bcp.2023.115983_b0110",
"volume": "17",
"year": "2023"
},
{
"article-title": "Hijacking SARS-CoV-2/ACE2 receptor interaction by natural and semi-synthetic steroidal agents acting on functional pockets on the receptor binding domain",
"author": "Carino",
"first-page": "846",
"journal-title": "BioRxiv",
"key": "10.1016/j.bcp.2023.115983_b0115",
"volume": "8",
"year": "2020"
},
{
"key": "10.1016/j.bcp.2023.115983_b0120",
"unstructured": "I. Chen, S. Cassaro, Physiology, Bile Acids., in: StatPearls, StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., California Northstate University College of Medicine UN California Irvine / Kaweah Delta, Treasure Island (FL), 2020."
},
{
"DOI": "10.1002/cphy.c120027",
"article-title": "Bile formation and secretion",
"author": "Boyer",
"doi-asserted-by": "crossref",
"first-page": "1035",
"journal-title": "Compr. Physiol.",
"key": "10.1016/j.bcp.2023.115983_b0125",
"volume": "3",
"year": "2013"
},
{
"DOI": "10.1016/j.cis.2019.102045",
"article-title": "Bile salts in digestion and transport of lipids",
"author": "Macierzanka",
"doi-asserted-by": "crossref",
"journal-title": "Adv. Colloid Interface Sci.",
"key": "10.1016/j.bcp.2023.115983_b0130",
"volume": "274",
"year": "2019"
},
{
"DOI": "10.2337/dc09-S355",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0135",
"unstructured": "B. Staels, V.A. Fonseca, Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration, Diab. Care, 32 Suppl 2 (2009) S237–S245. https://doi.org/10.2337/dc09-S355."
},
{
"DOI": "10.1111/imcb.12332",
"article-title": "Bile acids mediate signaling between microbiome and the immune system",
"author": "Liston",
"doi-asserted-by": "crossref",
"first-page": "349",
"journal-title": "Immunol. Cell Biol.",
"key": "10.1016/j.bcp.2023.115983_b0140",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1007/164_2019_238",
"article-title": "The pharmacology of bile acids and their receptors",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Handb. Exp. Pharmacol.",
"key": "10.1016/j.bcp.2023.115983_b0145",
"volume": "256",
"year": "2019"
},
{
"DOI": "10.1016/j.phrs.2015.12.007",
"article-title": "Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules",
"author": "Copple",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Pharmacol. Res.",
"key": "10.1016/j.bcp.2023.115983_b0150",
"volume": "104",
"year": "2016"
},
{
"DOI": "10.1146/annurev.biochem.72.121801.161712",
"article-title": "The enzymes, regulation, and genetics of bile acid synthesis",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "137",
"journal-title": "Annu. Rev. Biochem.",
"key": "10.1016/j.bcp.2023.115983_b0155",
"volume": "72",
"year": "2003"
},
{
"DOI": "10.1194/jlr.R900010-JLR200",
"article-title": "Bile acids: regulation of synthesis: thematic review series: bile acids",
"author": "Chiang",
"doi-asserted-by": "crossref",
"first-page": "1955",
"journal-title": "J. Lipid Res.",
"key": "10.1016/j.bcp.2023.115983_b0160",
"volume": "50",
"year": "2009"
},
{
"DOI": "10.1017/S1466252316000153",
"article-title": "Bacterial bile salt hydrolase: an intestinal microbiome target for enhanced animal health",
"author": "Geng",
"doi-asserted-by": "crossref",
"first-page": "148",
"journal-title": "Anim Heal. Res Rev.",
"key": "10.1016/j.bcp.2023.115983_b0165",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1194/jlr.R500013-JLR200",
"article-title": "Bile salt biotransformations by human intestinal bacteria",
"author": "Ridlon",
"doi-asserted-by": "crossref",
"first-page": "241",
"journal-title": "J. Lipid Res.",
"key": "10.1016/j.bcp.2023.115983_b0170",
"volume": "47",
"year": "2006"
},
{
"DOI": "10.1016/j.molmed.2015.09.001",
"article-title": "Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"first-page": "702",
"journal-title": "Trends Mol. Med.",
"key": "10.1016/j.bcp.2023.115983_b0175",
"volume": "21",
"year": "2015"
},
{
"DOI": "10.2165/00003088-197904050-00003",
"article-title": "Biliary excretion of drugs in man",
"author": "Rollins",
"doi-asserted-by": "crossref",
"first-page": "368",
"journal-title": "Clin. Pharmacokinet.",
"key": "10.1016/j.bcp.2023.115983_b0180",
"volume": "4",
"year": "1979"
},
{
"DOI": "10.1152/physrev.00010.2008",
"article-title": "Role of bile acids and bile acid receptors in metabolic regulation",
"author": "Lefebvre",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "Physiol. Rev.",
"key": "10.1016/j.bcp.2023.115983_b0185",
"volume": "89",
"year": "2009"
},
{
"DOI": "10.1016/S0006-291X(02)02550-0",
"article-title": "Identification of membrane-type receptor for bile acids (M-BAR)",
"author": "Maruyama",
"doi-asserted-by": "crossref",
"first-page": "714",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.bcp.2023.115983_b0190",
"volume": "298",
"year": "2002"
},
{
"DOI": "10.1016/S1097-2765(00)80348-2",
"article-title": "Endogenous bile acids are ligands for the nuclear receptor FXR/BAR",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "543",
"journal-title": "Mol. Cell.",
"key": "10.1016/j.bcp.2023.115983_b0195",
"volume": "3",
"year": "1999"
},
{
"DOI": "10.1126/science.284.5418.1365",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0200",
"unstructured": "D.J. Parks, S.G. Blanchard, R.K. Bledsoe, G. Chandra, T.G. Consler, S.A. Kliewer, J.B. Stimmel, T.M. Willson, A.M. Zavacki, D.D. Moore, J.M. Lehmann, Bile acids: natural ligands for an orphan nuclear receptor, Science (80-.). 284 (1999) 1365–1368. https://doi.org/10.1126/science.284.5418.1365."
},
{
"DOI": "10.1126/science.284.5418.1362",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0205",
"unstructured": "M. Makishima, A.Y. Okamoto, J.J. Repa, H. Tu, R.M. Learned, A. Luk, M. V Hull, K.D. Lustig, D.J. Mangelsdorf, B. Shan, Identification of a nuclear receptor for bile acids, Science (80-.). 284 (1999) 1362–1365. https://doi.org/10.1126/science.284.5418.1362."
},
{
"DOI": "10.1016/j.mce.2022.111650",
"article-title": "Immunomodulatory functions of FXR",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"journal-title": "Mol. Cell. Endocrinol.",
"key": "10.1016/j.bcp.2023.115983_b0210",
"volume": "551",
"year": "2022"
},
{
"DOI": "10.1016/j.livres.2021.08.003",
"article-title": "Bile acid activated receptors: Integrating immune and metabolic regulation in non-alcoholic fatty liver disease",
"author": "Biagioli",
"doi-asserted-by": "crossref",
"first-page": "119",
"journal-title": "Liver Res.",
"key": "10.1016/j.bcp.2023.115983_b0215",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1007/s10620-020-06715-3",
"article-title": "Bile Acid Signaling in Inflammatory Bowel Diseases",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"first-page": "674",
"journal-title": "Dig. Dis. Sci.",
"key": "10.1016/j.bcp.2023.115983_b0220",
"volume": "66",
"year": "2021"
},
{
"DOI": "10.1016/j.plipres.2021.101094",
"article-title": "Bile acids and their receptors in metabolic disorders",
"author": "Fiorucci",
"doi-asserted-by": "crossref",
"journal-title": "Prog. Lipid. Res.",
"key": "10.1016/j.bcp.2023.115983_b0225",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1126/science.1070477",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0230",
"unstructured": "M. Makishima, T.T. Lu, W. Xie, G.K. Whitfield, H. Domoto, R.M. Evans, M.R. Haussler, D.J. Mangelsdorf, Vitamin D receptor as an intestinal bile acid sensor, Science (80-.). 296 (2002) 1313–1316. https://doi.org/10.1126/science.1070477."
},
{
"DOI": "10.1073/pnas.051551698",
"article-title": "The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity",
"author": "Staudinger",
"doi-asserted-by": "crossref",
"first-page": "3369",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.bcp.2023.115983_b0235",
"volume": "98",
"year": "2001"
},
{
"DOI": "10.1093/toxsci/kfw054",
"article-title": "Activation of Constitutive Androstane Receptor (CAR) in Mice Results in Maintained Biliary Excretion of Bile Acids Despite a Marked Decrease of Bile Acids in Liver",
"author": "Lickteig",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "Toxicol. Sci.",
"key": "10.1016/j.bcp.2023.115983_b0240",
"volume": "151",
"year": "2016"
},
{
"DOI": "10.1002/hep.20784",
"article-title": "CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice",
"author": "Wagner",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Hepatology",
"key": "10.1016/j.bcp.2023.115983_b0245",
"volume": "42",
"year": "2005"
},
{
"DOI": "10.1194/jlr.R069286",
"article-title": "The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases",
"author": "Nagahashi",
"doi-asserted-by": "crossref",
"first-page": "1636",
"journal-title": "J. Lipid Res.",
"key": "10.1016/j.bcp.2023.115983_b0250",
"volume": "57",
"year": "2016"
},
{
"DOI": "10.1016/j.cell.2020.05.032",
"article-title": "Proteomic and Metabolomic Characterization of COVID-19 Patient Sera",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "59",
"journal-title": "Cell",
"key": "10.1016/j.bcp.2023.115983_b0255",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1038/s41598-022-05667-0",
"article-title": "Metabolomics study of COVID-19 patients in four different clinical stages",
"author": "Valdés",
"doi-asserted-by": "crossref",
"first-page": "1650",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.bcp.2023.115983_b0260",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/v15061231",
"article-title": "Gastrointestinal manifestations of SARS-CoV-2: transmission, pathogenesis, immunomodulation, microflora dysbiosis, and clinical implications",
"author": "Durairajan",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/j.bcp.2023.115983_b0265",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19",
"author": "Yeoh",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "10.1016/j.bcp.2023.115983_b0270",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1053/j.gastro.2020.05.048",
"article-title": "Alterations in gut microbiota of patients with COVID-19 during time of hospitalization",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "944",
"journal-title": "Gastroenterology",
"key": "10.1016/j.bcp.2023.115983_b0275",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2017.12.024",
"article-title": "An “Old” protein with a new story: Coronavirus endoribonuclease is important for evading host antiviral defenses",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "157",
"journal-title": "Virology",
"key": "10.1016/j.bcp.2023.115983_b0280",
"volume": "517",
"year": "2018"
},
{
"DOI": "10.1073/pnas.2123208119",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0285",
"unstructured": "C.E. Comar, C.J. Otter, J. Pfannenstiel, E. Doerger, D.M. Renner, L.H. Tan, S. Perlman, N.A. Cohen, A.R. Fehr, S.R. Weiss, MERS-CoV endoribonuclease and accessory proteins jointly evade host innate immunity during infection of lung and nasal epithelial cells, Proc. Natl. Acad. Sci. U. S. A. 119 (2022) e2123208119. https://doi.org/10.1073/pnas.2123208119."
},
{
"DOI": "10.3389/fcimb.2022.896504",
"article-title": "Antibiotic-Induced Primary Biles Inhibit SARS-CoV-2 Endoribonuclease Nsp15 Activity in Mouse Gut",
"author": "Ma",
"doi-asserted-by": "crossref",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "10.1016/j.bcp.2023.115983_b0290",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1080/07391102.2020.1868339",
"article-title": "Virtual repurposing of ursodeoxycholate and chenodeoxycholate as lead candidates against SARS-Cov2-Envelope protein: A molecular dynamics investigation",
"author": "Yadav",
"doi-asserted-by": "crossref",
"first-page": "5147",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.bcp.2023.115983_b0295",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1080/07391102.2022.2133010",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0300",
"unstructured": "G. Rocha Aguiar, T. Leda Gomes de Lemos, R. Braz-Filho, A. Marques da Fonseca, E. Silva Marinho, P.R. Vasconcelos Ribeiro, K. Marques Canuto, F.J. Queiroz Monte, Synthesis and in silico study of chenodeoxycholic acid and its analogues as an alternative inhibitor of spike glycoprotein of SARS-CoV-2., J. Biomol. Struct. Dyn. 41 (2023) 8334–8348. https://doi.org/10.1080/07391102.2022.2133010."
},
{
"DOI": "10.1038/s41598-021-01705-5",
"article-title": "Interaction of Spike protein and lipid membrane of SARS-CoV-2 with Ursodeoxycholic acid, an in-silico analysis",
"author": "Rodal Canales",
"doi-asserted-by": "crossref",
"first-page": "22288",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.bcp.2023.115983_b0305",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2022.113021",
"article-title": "Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells",
"author": "Thuy",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.bcp.2023.115983_b0310",
"volume": "150",
"year": "2022"
},
{
"DOI": "10.3390/v12111325",
"article-title": "Target-Centered Drug Repurposing Predictions of Human Angiotensin-Converting Enzyme 2 (ACE2) and Transmembrane Protease Serine Subtype 2 (TMPRSS2) Interacting Approved Drugs for Coronavirus Disease 2019 (COVID-19) Treatment through a Drug-Target Interact",
"author": "Choi",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/j.bcp.2023.115983_b0315",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1002/emmm.201000080",
"article-title": "Angiotensin II revisited: new roles in inflammation, immunology and aging",
"author": "Benigni",
"doi-asserted-by": "crossref",
"first-page": "247",
"journal-title": "EMBO Mol. Med.",
"key": "10.1016/j.bcp.2023.115983_b0320",
"volume": "2",
"year": "2010"
},
{
"DOI": "10.1124/pr.118.017129",
"article-title": "Novel Therapeutic Approaches Targeting the Renin-Angiotensin System and Associated Peptides in Hypertension and Heart Failure",
"author": "Arendse",
"doi-asserted-by": "crossref",
"first-page": "539",
"journal-title": "Pharmacol. Rev.",
"key": "10.1016/j.bcp.2023.115983_b0325",
"volume": "71",
"year": "2019"
},
{
"DOI": "10.1021/acs.jcim.1c01126",
"article-title": "Discovery of Bile Acid Derivatives as Potent ACE2 Activators by Virtual Screening and Essential Dynamics",
"author": "Fiorillo",
"doi-asserted-by": "crossref",
"first-page": "196",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.bcp.2023.115983_b0330",
"volume": "62",
"year": "2022"
},
{
"DOI": "10.3390/cells11071187",
"article-title": "GLP-1 Mediates Regulation of Colonic ACE2 Expression by the Bile Acid Receptor GPBAR1 in Inflammation",
"author": "Biagioli",
"doi-asserted-by": "crossref",
"journal-title": "Cells",
"key": "10.1016/j.bcp.2023.115983_b0335",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1021/jm501273r",
"article-title": "Exploitation of cholane scaffold for the discovery of potent and selective farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (GP-BAR1) ligands",
"author": "Festa",
"doi-asserted-by": "crossref",
"first-page": "8477",
"journal-title": "J. Med. Chem.",
"key": "10.1016/j.bcp.2023.115983_b0340",
"volume": "57",
"year": "2014"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"doi-asserted-by": "crossref",
"key": "10.1016/j.bcp.2023.115983_b0345",
"unstructured": "[69] T. Brevini, M. Maes, G.J. Webb, B. V John, C.D. Fuchs, G. Buescher, L. Wang, C. Griffiths, M.L. Brown, W.E. 3rd Scott, P. Pereyra-Gerber, W.T.H. Gelson, S. Brown, S. Dillon, D. Muraro, J. Sharp, M. Neary, H. Box, L. Tatham, J. Stewart, P. Curley, H. Pertinez, S. Forrest, P. Mlcochova, S.S. Varankar, M. Darvish-Damavandi, V.L. Mulcahy, R.E. Kuc, T.L. Williams, J.A. Heslop, D. Rossetti, O.C. Tysoe, V. Galanakis, M. Vila-Gonzalez, T.W.M. Crozier, J. Bargehr, S. Sinha, S.S. Upponi, C. Fear, L. Swift, K. Saeb-Parsy, S.E. Davies, A. Wester, H. Hagström, E. Melum, D. Clements, P. Humphreys, J. Herriott, E. Kijak, H. Cox, C. Bramwell, A. Valentijn, C.J.R. Illingworth, B. Dahman, D.R. Bastaich, R.D. Ferreira, T. Marjot, E. Barnes, A.M. Moon, A.S. 4th Barritt, R.K. Gupta, S. Baker, A.P. Davenport, G. Corbett, V.G. Gorgoulis, S.J.A. Buczacki, J.-H. Lee, N.J. Matheson, M. Trauner, A.J. Fisher, P. Gibbs, A.J. Butler, C.J.E. Watson, G.F. Mells, G. Dougan, A. Owen, A.W. Lohse, L. Vallier, F. Sampaziotis, FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2., Nature. (2022). https://doi.org/10.1038/s41586-022-05594-0."
},
{
"article-title": "Ursodeoxycholic acid is a GPBAR1 agonist and resets liver/intestinal FXR signaling in a model of diet-induced dysbiosis and NASH",
"author": "Carino",
"first-page": "1422",
"journal-title": "Biochim. Biophys. Acta Mol. Cell. Biol. Lipids",
"key": "10.1016/j.bcp.2023.115983_b0350",
"volume": "2019",
"year": "1864"
},
{
"DOI": "10.1016/j.bcp.2009.06.026",
"article-title": "The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease",
"author": "Mencarelli",
"doi-asserted-by": "crossref",
"first-page": "1214",
"journal-title": "Biochem. Pharmacol.",
"key": "10.1016/j.bcp.2023.115983_b0355",
"volume": "78",
"year": "2009"
},
{
"DOI": "10.1093/ecco-jcc/jjaa185",
"article-title": "Intestinal Receptor of SARS-CoV-2 in Inflamed IBD Tissue Seems Downregulated by HNF4A in Ileum and Upregulated by Interferon Regulating Factors in Colon",
"author": "Verstockt",
"doi-asserted-by": "crossref",
"first-page": "485",
"journal-title": "J. Crohns Colitis",
"key": "10.1016/j.bcp.2023.115983_b0360",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1053/j.gastro.2020.10.041",
"article-title": "Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease",
"author": "Potdar",
"doi-asserted-by": "crossref",
"first-page": "809",
"journal-title": "Gastroenterology",
"key": "10.1016/j.bcp.2023.115983_b0365",
"volume": "160",
"year": "2020"
},
{
"article-title": "Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts., Liver Int",
"author": "Marrone",
"journal-title": "Off. J. Int. Assoc. Study Liver.",
"key": "10.1016/j.bcp.2023.115983_b0370",
"year": "2023"
},
{
"DOI": "10.3390/v15081738",
"article-title": "Ursodeoxycholic Acid does not improve COVID-19 outcome in hospitalized patients",
"author": "Colapietro",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/j.bcp.2023.115983_b0375",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/cells10061281",
"article-title": "Bile acids activated receptors in inflammatory bowel disease",
"author": "Biagioli",
"doi-asserted-by": "crossref",
"journal-title": "Cells",
"key": "10.1016/j.bcp.2023.115983_b0380",
"volume": "10",
"year": "2021"
},
{
"article-title": "Farnesoid X receptor suppresses constitutive androstane receptor activity at the multidrug resistance protein-4 promoter",
"author": "Renga",
"first-page": "157",
"journal-title": "Biochim. Biophys. Acta",
"key": "10.1016/j.bcp.2023.115983_b0385",
"volume": "2011",
"year": "1809"
},
{
"DOI": "10.4049/jimmunol.0803978",
"article-title": "The bile acid receptor FXR is a modulator of intestinal innate immunity",
"author": "Vavassori",
"doi-asserted-by": "crossref",
"first-page": "6251",
"journal-title": "J. Immunol.",
"key": "10.1016/j.bcp.2023.115983_b0390",
"volume": "183",
"year": "2009"
},
{
"DOI": "10.1096/fj.201801373RR",
"article-title": "Agonism for the bile acid receptor GPBAR1 reverses liver and vascular damage in a mouse model of steatohepatitis",
"author": "Carino",
"doi-asserted-by": "crossref",
"first-page": "2809",
"journal-title": "FASEB J.",
"key": "10.1016/j.bcp.2023.115983_b0395",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1038/s41586-019-1785-z",
"article-title": "Bile acid metabolites control T(H)17 and T(reg) cell differentiation",
"author": "Hang",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "Nature",
"key": "10.1016/j.bcp.2023.115983_b0400",
"volume": "576",
"year": "2019"
},
{
"DOI": "10.1111/joim.13630",
"article-title": "Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis",
"author": "John",
"doi-asserted-by": "crossref",
"first-page": "636",
"journal-title": "J. Intern. Med.",
"key": "10.1016/j.bcp.2023.115983_b0405",
"volume": "293",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID-19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1033",
"journal-title": "Lancet (Lond., Engl.)",
"key": "10.1016/j.bcp.2023.115983_b0410",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.arcmed.2020.04.019",
"article-title": "Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection",
"author": "Delgado-Roche",
"doi-asserted-by": "crossref",
"first-page": "384",
"journal-title": "Arch. Med. Res.",
"key": "10.1016/j.bcp.2023.115983_b0415",
"volume": "51",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0180673",
"article-title": "Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "e0180673",
"journal-title": "PLoS One",
"key": "10.1016/j.bcp.2023.115983_b0420",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1016/S0006-2952(02)01391-6",
"article-title": "Antioxidant properties of ursodeoxycholic acid",
"author": "Lapenna",
"doi-asserted-by": "crossref",
"first-page": "1661",
"journal-title": "Biochem. Pharmacol.",
"key": "10.1016/j.bcp.2023.115983_b0425",
"volume": "64",
"year": "2002"
},
{
"DOI": "10.1016/j.mehy.2020.109897",
"article-title": "Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm",
"author": "Abdulrab",
"doi-asserted-by": "crossref",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.bcp.2023.115983_b0430",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(04)16680-4",
"article-title": "Antimalarial combinations",
"author": "Kremsner",
"doi-asserted-by": "crossref",
"first-page": "285",
"journal-title": "Lancet (Lond., Engl.)",
"key": "10.1016/j.bcp.2023.115983_b0435",
"volume": "364",
"year": "2004"
},
{
"DOI": "10.1096/fj.202200171R",
"article-title": "Dihydroartemisinin promoted FXR expression independent of YAP1 in hepatocellular carcinoma",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "e22361",
"journal-title": "FASEB J.",
"key": "10.1016/j.bcp.2023.115983_b0440",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2021.751772",
"article-title": "Immunoregulation by Artemisinin and Its Derivatives: A New Role for Old Antimalarial Drugs",
"author": "Qiu",
"doi-asserted-by": "crossref",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.bcp.2023.115983_b0445",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1021/acsinfecdis.0c00522",
"article-title": "Anti-SARS-CoV-2 Potential of Artemisinins In Vitro",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "2524",
"journal-title": "ACS Infect. Dis.",
"key": "10.1016/j.bcp.2023.115983_b0450",
"volume": "6",
"year": "2020"
},
{
"author": "Berberine",
"first-page": "175",
"journal-title": "Altern. Med. Rev.",
"key": "10.1016/j.bcp.2023.115983_b0455",
"volume": "5",
"year": "2000"
},
{
"DOI": "10.3389/fphar.2021.750826",
"article-title": "Berberine Alleviates Non-alcoholic Steatohepatitis Through Modulating Gut Microbiota Mediated Intestinal FXR Activation",
"author": "Shu",
"doi-asserted-by": "crossref",
"journal-title": "Front. Pharmacol.",
"key": "10.1016/j.bcp.2023.115983_b0460",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1007/s13659-020-00253-1",
"article-title": "Recognition of Natural Products as Potential Inhibitors of COVID-19 Main Protease (Mpro): In-Silico Evidences",
"author": "Narkhede",
"doi-asserted-by": "crossref",
"first-page": "297",
"journal-title": "Nat. Products Bioprospect.",
"key": "10.1016/j.bcp.2023.115983_b0465",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s10787-022-01080-1",
"article-title": "The role of berberine in Covid-19: potential adjunct therapy",
"author": "Babalghith",
"doi-asserted-by": "crossref",
"first-page": "2003",
"journal-title": "Inflammopharmacology",
"key": "10.1016/j.bcp.2023.115983_b0470",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1016/j.phytochem.2006.06.020",
"article-title": "Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives",
"author": "Nagle",
"doi-asserted-by": "crossref",
"first-page": "1849",
"journal-title": "Phytochemistry",
"key": "10.1016/j.bcp.2023.115983_b0475",
"volume": "67",
"year": "2006"
},
{
"DOI": "10.1186/s13578-021-00680-8",
"article-title": "Epigallocatechin gallate from green tea effectively blocks infection of SARS-CoV-2 and new variants by inhibiting spike binding to ACE2 receptor",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "168",
"journal-title": "Cell Biosci.",
"key": "10.1016/j.bcp.2023.115983_b0480",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0271112",
"article-title": "Epigallocatechin gallate (EGCG) attenuates severe acute respiratory coronavirus disease 2 (SARS-CoV-2) infection by blocking the interaction of SARS-CoV-2 spike protein receptor-binding domain to human angiotensin-converting enzyme 2",
"author": "Ohishi",
"doi-asserted-by": "crossref",
"first-page": "e0271112",
"journal-title": "PLoS One",
"key": "10.1016/j.bcp.2023.115983_b0485",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1080/07391102.2020.1779818",
"article-title": "Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study",
"author": "Ghosh",
"doi-asserted-by": "crossref",
"first-page": "4362",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.bcp.2023.115983_b0490",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.3390/molecules26051200",
"article-title": "EGCG, a Green Tea Catechin, as a Potential Therapeutic Agent for Symptomatic and Asymptomatic SARS-CoV-2 Infection",
"author": "Chourasia",
"doi-asserted-by": "crossref",
"journal-title": "Molecules",
"key": "10.1016/j.bcp.2023.115983_b0495",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1021/acs.jmedchem.7b00907",
"article-title": "Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH)",
"author": "Tully",
"doi-asserted-by": "crossref",
"first-page": "9960",
"journal-title": "J. Med. Chem.",
"key": "10.1016/j.bcp.2023.115983_b0500",
"volume": "60",
"year": "2017"
},
{
"DOI": "10.1021/acsinfecdis.1c00629",
"article-title": "Drug-repurposing screening identified tropifexor as a SARS-CoV-2 papain-like protease inhibitor",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "1022",
"journal-title": "ACS Infect. Dis.",
"key": "10.1016/j.bcp.2023.115983_b0505",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1007/978-3-319-41342-6_15",
"article-title": "Guggulsterone and its role in chronic diseases",
"author": "Yamada",
"doi-asserted-by": "crossref",
"first-page": "329",
"journal-title": "Adv. Exp. Med. Biol.",
"key": "10.1016/j.bcp.2023.115983_b0510",
"volume": "929",
"year": "2016"
},
{
"DOI": "10.3390/molecules27238287",
"article-title": "Computational bioprospecting guggulsterone against ADP ribose phosphatase of SARS-CoV-2",
"author": "Kciuk",
"doi-asserted-by": "crossref",
"journal-title": "Molecules",
"key": "10.1016/j.bcp.2023.115983_b0515",
"volume": "27",
"year": "2022"
},
{
"article-title": "Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children",
"author": "Liu",
"first-page": "1950",
"journal-title": "Liver Int. Off. J. Int. Assoc. Study Liver.",
"key": "10.1016/j.bcp.2023.115983_b0520",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.3389/fcimb.2023.1178590",
"article-title": "Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1178590",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "10.1016/j.bcp.2023.115983_b0525",
"volume": "13",
"year": "2023"
}
],
"reference-count": 105,
"references-count": 105,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0006295223005762"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"Biochemistry"
],
"subtitle": [],
"title": "Bile acids and bile acid activated receptors in the treatment of Covid-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}