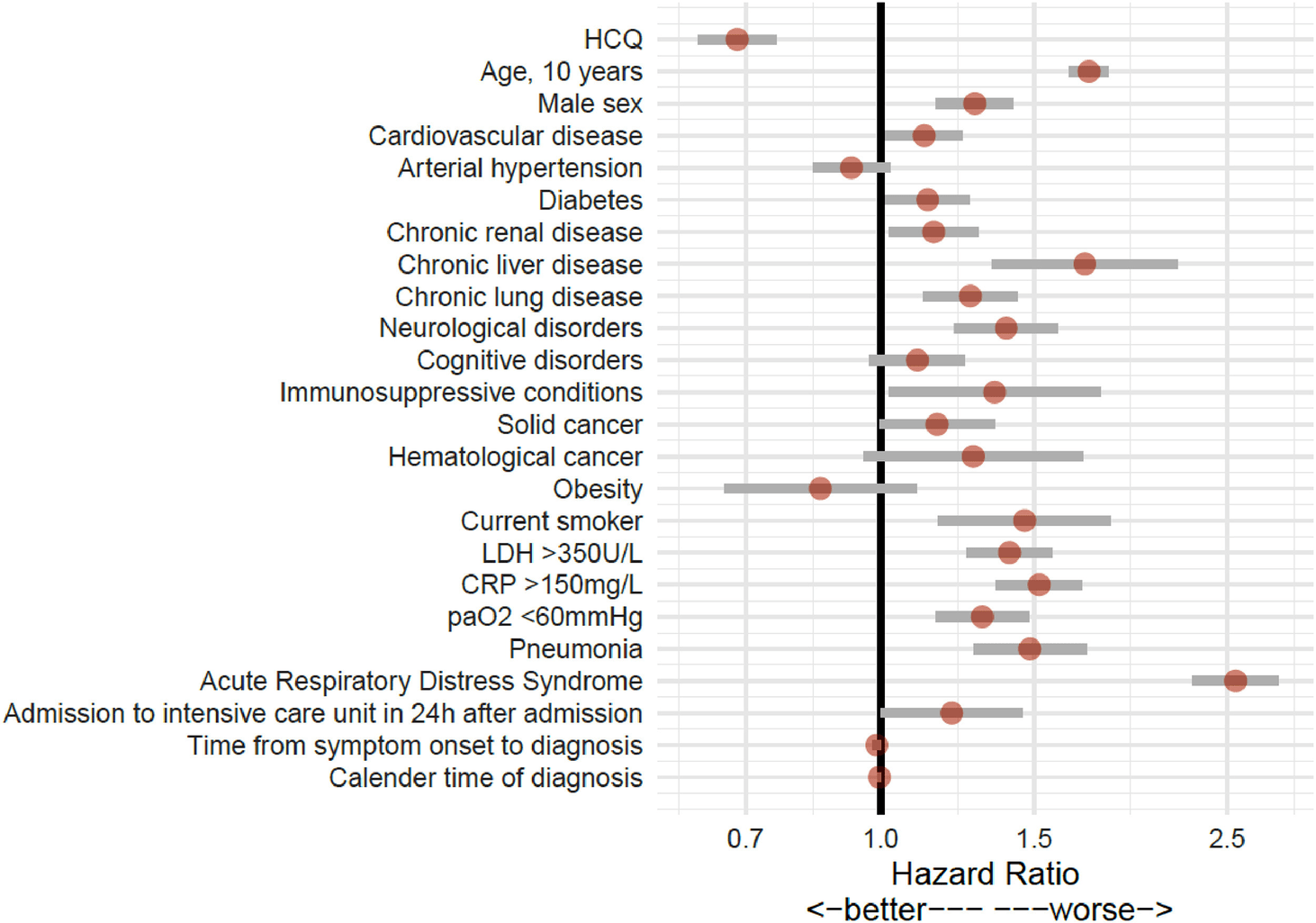
Low-dose Hydroxychloroquine Therapy and Mortality in Hospitalized Patients with COVID-19: A Nationwide Observational Study of 8075 Participants
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106144, Aug 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 8,075 hospitalized patients, 4,542 low-dose HCQ, 3,533 control. 35% lower mortality for HCQ (17.7% vs. 27.1%), adjusted HR 0.68 [0.62-0.76]. Low-dose HCQ monotherapy was independently associated with lower mortality in hospitalized patients.
Patients exposed to others therapies (TCZ, AZ, LPV/RTV) were excluded.
Statistical analysis was performed by an independent group. Calendar time of prescription and immortal time bias was taken into account. Corticosteroids prescriptions was low in both groups.
|
risk of death, 32.0% lower, HR 0.68, p < 0.001, treatment 804 of 4,542 (17.7%), control 957 of 3,533 (27.1%), NNT 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Catteau et al., 24 Aug 2020, retrospective, database analysis, Belgium, peer-reviewed, 11 authors, average treatment delay 5.0 days.
Abstract: International Journal of Antimicrobial Agents 56 (2020) 106144
Contents lists available at ScienceDirect
International Journal of Antimicrobial Agents
journal homepage: www.elsevier.com/locate/ijantimicag
Low-dose hydroxychloroquine therapy and mortality in hospitalised
patients with COVID-19: a nationwide observational study of 8075
participants
Lucy Catteau a,1, Nicolas Dauby b,c,d,1,∗, Marion Montourcy a, Emmanuel Bottieau e,
Joris Hautekiet a,f, Els Goetghebeur f, Sabrina van Ierssel g, Els Duysburgh a, Herman Van
Oyen a,h, Chloé Wyndham-Thomas a, Dominique Van Beckhoven a , Belgian Collaborative
Group on COVID-19 Hospital Surveillance2
a
Department of Epidemiology and public health, Sciensano, Brussels, Belgium
Department of Infectious Diseases, CHU Saint-Pierre, Brussels, Belgium
c
Institute for Medical Immunology, Université Libre de Bruxelles (ULB), Brussels, Belgium
d
Environmental Health Research Centre, Public Health School, Université Libre de Bruxelles (ULB), Brussels, Belgium
e
Department of Clinical Sciences, Institute of Tropical Medicine, Antwerp, Belgium
f
Department of Applied Mathematics, Computer Science and Statistics, Ghent University, Ghent, Belgium
g
Department of General Internal Medicine, Infectious Diseases and Tropical Medicine, University Hospital Antwerp (UZA), Edegem, Belgium
h
Public Health and Primary Care, Gent University, Gent, Belgium
b
a r t i c l e
Keywords:
Hydroxychloroquine
COVID-19
SARS-CoV-2
Mortality
Observational study
i n f o
a b s t r a c t
Hydroxychloroquine (HCQ) has been largely used and investigated as therapy for COVID-19 across various settings at a total dose usually ranging from 2400 mg to 9600 mg. In Belgium, off-label use of
low-dose HCQ (total 2400 mg over 5 days) was recommended for hospitalised patients with COVID-19.
We conducted a retrospective analysis of in-hospital mortality in the Belgian national COVID-19 hospital
surveillance data. Patients treated either with HCQ monotherapy and supportive care (HCQ group) were
compared with patients treated with supportive care only (no-HCQ group) using a competing risks proportional hazards regression with discharge alive as competing risk, adjusted for demographic and clinical features with robust standard errors. Of 8075 patients with complete discharge data on 24 May 2020
and diagnosed before 1 May 2020, 4542 received HCQ in monotherapy and 3533 were in the no-HCQ
group. Death was reported in 804/4542 (17.7%) and 957/3533 (27.1%), respectively. In the multivariable
analysis, mortality was lower in the HCQ group compared with the no-HCQ group [adjusted hazard ratio
(aHR) = 0.684, 95% confidence interval (CI) 0.617–0.758]. Compared with the no-HCQ group, mortality
in the HCQ group was reduced both in patients diagnosed ≤5 days (n = 3975) and >5 days (n = 3487)
after symptom onset [aHR = 0.701 (95% CI 0.617–0.796) and aHR = 0.647 (95% CI 0.525–0.797), respectively]. Compared with supportive care only, low-dose HCQ monotherapy was independently associated
with lower mortality in hospitalised patients with COVID-19 diagnosed and treated early or later after
symptom onset.
© 2020 Elsevier Ltd and International Society of Antimicrobial Chemotherapy. All rights reserved.
DOI record:
{
"DOI": "10.1016/j.ijantimicag.2020.106144",
"ISSN": [
"0924-8579"
],
"URL": "http://dx.doi.org/10.1016/j.ijantimicag.2020.106144",
"alternative-id": [
"S0924857920303423"
],
"article-number": "106144",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Antimicrobial Agents"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijantimicag.2020.106144"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier Ltd and International Society of Antimicrobial Chemotherapy. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4695-4275",
"affiliation": [],
"authenticated-orcid": false,
"family": "Catteau",
"given": "Lucy",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-7697-6849",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dauby",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montourcy",
"given": "Marion",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bottieau",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hautekiet",
"given": "Joris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goetghebeur",
"given": "Els",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Ierssel",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duysburgh",
"given": "Els",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van Oyen",
"given": "Herman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wyndham-Thomas",
"given": "Chloé",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van Beckhoven",
"given": "Dominique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bafort",
"given": "Kristof",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belkhir",
"given": "Leïla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bossuyt",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caprasse",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colombie",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Munter",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deblonde",
"given": "Jessika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delmarcelle",
"given": "Didier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delvallee",
"given": "Mélanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Demeester",
"given": "Rémy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dugernier",
"given": "Thierry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holemans",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kerzmann",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yves Machurot",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Minette",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Minon",
"given": "Jean-Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mokrane",
"given": "Saphia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nachtergal",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noirhomme",
"given": "Séverine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piérard",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossi",
"given": "Camelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schirvel",
"given": "Carole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sermijn",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Staelens",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Triest",
"given": "Filip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goethem",
"given": "Nina Van",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Praet",
"given": "Jens Van",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vanhoenacker",
"given": "Anke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verstraete",
"given": "Roeland",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Willems",
"given": "Elise",
"sequence": "additional"
}
],
"container-title": "International Journal of Antimicrobial Agents",
"container-title-short": "International Journal of Antimicrobial Agents",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
8,
24
]
],
"date-time": "2020-08-24T15:36:25Z",
"timestamp": 1598283385000
},
"deposited": {
"date-parts": [
[
2021,
1,
14
]
],
"date-time": "2021-01-14T23:44:24Z",
"timestamp": 1610667864000
},
"funder": [
{
"DOI": "10.13039/501100003441",
"award": [
"BC-07507"
],
"doi-asserted-by": "publisher",
"name": "Universitair Ziekenhuis Gent"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
8
]
],
"date-time": "2024-03-08T02:03:01Z",
"timestamp": 1709863381479
},
"is-referenced-by-count": 96,
"issue": "4",
"issued": {
"date-parts": [
[
2020,
10
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2020,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T00:00:00Z",
"timestamp": 1601510400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857920303423?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857920303423?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "106144",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.bbrc.2004.08.085",
"article-title": "In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine",
"author": "Keyaerts",
"doi-asserted-by": "crossref",
"first-page": "264",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.1016/j.ijantimicag.2020.106144_bib0001",
"volume": "323",
"year": "2004"
},
{
"DOI": "10.1186/1743-422X-2-69",
"article-title": "Chloroquine is a potent inhibitor of SARS coronavirus infection and spread",
"author": "Vincent",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Virol J",
"key": "10.1016/j.ijantimicag.2020.106144_bib0002",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/j.ijantimicag.2020.106144_bib0003",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "16",
"journal-title": "Cell Discov",
"key": "10.1016/j.ijantimicag.2020.106144_bib0004",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa546",
"article-title": "Chloroquine inhibits the release of inflammatory cytokines by human lung explants",
"author": "Grassin-Delyle",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0005",
"year": "2020"
},
{
"DOI": "10.1016/j.immuni.2020.05.002",
"article-title": "Immunology of COVID-19: current state of the science",
"author": "Vabret",
"doi-asserted-by": "crossref",
"first-page": "910",
"journal-title": "Immunity",
"key": "10.1016/j.ijantimicag.2020.106144_bib0006",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1038/s41584-020-0372-x",
"article-title": "Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology",
"author": "Schrezenmeier",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Nat Rev Rheumatol",
"key": "10.1016/j.ijantimicag.2020.106144_bib0007",
"volume": "16",
"year": "2020"
},
{
"key": "10.1016/j.ijantimicag.2020.106144_bib0008",
"unstructured": "Sciensano Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. Sciensano; 2020. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf."
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0009",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa394",
"article-title": "Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients",
"author": "Perinel",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0010",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with COVID-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"first-page": "2411",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijantimicag.2020.106144_bib0011",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijantimicag.2020.106144_bib0012",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1844",
"article-title": "Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: observational comparative study using routine care data",
"author": "Mahévas",
"doi-asserted-by": "crossref",
"first-page": "m1844",
"journal-title": "BMJ",
"key": "10.1016/j.ijantimicag.2020.106144_bib0013",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.8630",
"article-title": "Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State",
"author": "Rosenberg",
"doi-asserted-by": "crossref",
"first-page": "2493",
"journal-title": "JAMA",
"key": "10.1016/j.ijantimicag.2020.106144_bib0014",
"volume": "323",
"year": "2020"
},
{
"article-title": "Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe COVID-19 in a French university hospital",
"author": "Paccoud",
"first-page": "2493",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0015",
"volume": "323",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France",
"author": "Sbidian",
"journal-title": "medRxiv",
"key": "10.1016/j.ijantimicag.2020.106144_bib0016",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101791",
"article-title": "Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis",
"author": "Lagier",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0017",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1007/s11606-020-05983-z",
"article-title": "Risk factors for mortality in patients with COVID-19 in New York City",
"author": "Mikami",
"doi-asserted-by": "crossref",
"journal-title": "J Gen Intern Med",
"key": "10.1016/j.ijantimicag.2020.106144_bib0018",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"article-title": "Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "396",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0019",
"volume": "97",
"year": "2020"
},
{
"article-title": "Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial",
"author": "Horby",
"journal-title": "medRxiv",
"key": "10.1016/j.ijantimicag.2020.106144_bib0020",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2020.00225",
"article-title": "Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context",
"author": "Mertens",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Front Med",
"key": "10.1016/j.ijantimicag.2020.106144_bib0021",
"volume": "7",
"year": "2020"
},
{
"article-title": "mice: Multivariate Imputation by Chained Equations in R",
"author": "van Buuren",
"first-page": "1",
"journal-title": "J Stat Softw",
"key": "10.1016/j.ijantimicag.2020.106144_bib0022",
"volume": "45",
"year": "2011"
},
{
"article-title": "ipw : an R package for inverse probability weighting",
"author": "van der Wal",
"journal-title": "J Stat Softw",
"key": "10.1016/j.ijantimicag.2020.106144_bib0023",
"volume": "43",
"year": "2011"
},
{
"DOI": "10.1002/sim.6777",
"article-title": "Comparisons of the performance of different statistical tests for time-to-event analysis with confounding factors: practical illustrations in kidney transplantation",
"author": "Le Borgne",
"doi-asserted-by": "crossref",
"first-page": "1103",
"journal-title": "Stat Med",
"key": "10.1016/j.ijantimicag.2020.106144_bib0024",
"volume": "35",
"year": "2016"
},
{
"author": "Sciensano",
"key": "10.1016/j.ijantimicag.2020.106144_bib0025",
"series-title": "COVID-19 — Bulletin épidémiologique du 25 mai 2020",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1985",
"article-title": "Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study",
"author": "Docherty",
"doi-asserted-by": "crossref",
"first-page": "m1985",
"journal-title": "BMJ",
"key": "10.1016/j.ijantimicag.2020.106144_bib0026",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "JAMA",
"key": "10.1016/j.ijantimicag.2020.106144_bib0027",
"volume": "323",
"year": "2020"
},
{
"article-title": "Dexamethasone in hospitalized patients with COVID-19—preliminary report",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijantimicag.2020.106144_bib0028",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/j.ijantimicag.2020.106144_bib0029",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06062-x",
"article-title": "High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study",
"author": "Helms",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "Intensive Care Med",
"key": "10.1016/j.ijantimicag.2020.106144_bib0030",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1055/s-0040-1709650",
"article-title": "D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis",
"author": "Lippi",
"doi-asserted-by": "crossref",
"first-page": "876",
"journal-title": "Thromb Haemost",
"key": "10.1016/j.ijantimicag.2020.106144_bib0031",
"volume": "120",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.06.095",
"article-title": "An observational cohort study of hydroxychloroquine and azithromycin for COVID-19: (can't get no) satisfaction",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "216",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0032",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104762",
"article-title": "Of chloroquine and COVID-19",
"author": "Touret",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ijantimicag.2020.106144_bib0033",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa623",
"article-title": "Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients",
"author": "Fan",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0034",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine in COVID-19 patients: what still needs to be known about the kinetics",
"author": "Martin-Blondel",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijantimicag.2020.106144_bib0035",
"year": "2020"
},
{
"article-title": "Antiviral treatment of SARS-CoV-2-infected hamsters reveals a weak effect of favipiravir and a complete lack of effect for hydroxychloroquine",
"author": "Kaptein",
"journal-title": "bioRxiv",
"key": "10.1016/j.ijantimicag.2020.106144_bib0036",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2558-4",
"article-title": "Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in non-human primates",
"author": "Maisonnasse",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "10.1016/j.ijantimicag.2020.106144_bib0037",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"article-title": "The trinity of COVID-19: immunity, inflammation and intervention",
"author": "Tay",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "Nat Rev Immunol",
"key": "10.1016/j.ijantimicag.2020.106144_bib0038",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1093/rheumatology/kei282",
"article-title": "Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "703",
"journal-title": "Rheumatology",
"key": "10.1016/j.ijantimicag.2020.106144_bib0039",
"volume": "45",
"year": "2006"
},
{
"DOI": "10.6061/clinics/2013(06)07",
"article-title": "Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients",
"author": "Silva",
"doi-asserted-by": "crossref",
"first-page": "766",
"journal-title": "Clinics (Sao Paulo)",
"key": "10.1016/j.ijantimicag.2020.106144_bib0040",
"volume": "68",
"year": "2013"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106078",
"article-title": "A pharmacological perspective of chloroquine in SARS-CoV-2 infection",
"author": "Oscanoa",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijantimicag.2020.106144_bib0041",
"year": "2020"
},
{
"DOI": "10.1038/d41586-020-01695-w",
"article-title": "High-profile coronavirus retractions raise concerns about data oversight",
"author": "Ledford",
"doi-asserted-by": "crossref",
"first-page": "160",
"journal-title": "Nature",
"key": "10.1016/j.ijantimicag.2020.106144_bib0042",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"article-title": "Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial",
"author": "Borba",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ijantimicag.2020.106144_bib0043",
"volume": "3",
"year": "2020"
},
{
"article-title": "Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study",
"author": "Lane",
"journal-title": "medRxiv",
"key": "10.1016/j.ijantimicag.2020.106144_bib0044",
"year": "2020"
},
{
"key": "10.1016/j.ijantimicag.2020.106144_bib0045",
"series-title": "Summary adverse drug reactions in corona virus infection",
"year": "2020"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0924857920303423"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "56"
}
