Effects of Tocilizumab in Critically Ill Patients With COVID-19: A Quasi-Experimental Study
et al., medRxiv, doi:10.1101/2020.07.13.20149328, Jul 2020
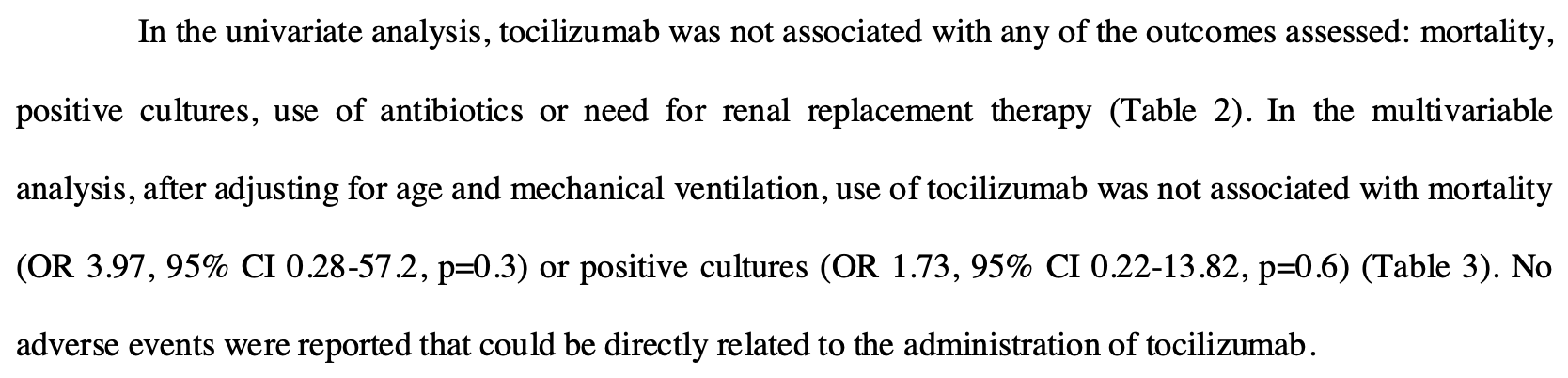
Retrospective 53 critically ill COVID-19 patients showing no difference in mortality, need for renal replacement therapy, use of antibiotics, or positive cultures with tocilizumab treatment.
|
risk of death, 165.6% higher, RR 2.66, p = 0.30, treatment 5 of 29 (17.2%), control 4 of 24 (16.7%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Carvalho et al., 15 Jul 2020, retrospective, Brazil, preprint, 6 authors, study period 21 March, 2020 - 31 May, 2020.
Contact: torcortes@yahoo.com.br.
Effects of Tocilizumab in Critically Ill Patients With COVID-19: A Quasi-Experimental Study
doi:10.1101/2020.07.13.20149328
Objectives: Critically ill patients with COVID-19 may suffer from a cytokine release syndrome (CRS) characterized by remarkably high levels of interleukin 6 (IL-6). We assessed the effects of tocilizumab, an IL-6 receptor antagonist, on intra-hospital mortality and development of positive cultures in patients with COVID-19 admitted to the ICU.
Design: Patients with COVID 19 admitted in the ICU who were treated with tocilizumab plus standard care were enrolled and compared to controls.
Setting
COVID-19 severe disease
Patients Patients with severe COVID-19 disease admitted in the ICU.
Interventions Tocilizumab 400 mg IV two doses. Standard and intensive medical care as per institutional clinical protocol.
Measures and Main Results Main outcome: 1) intra-hospital mortality; Secondary Outcomes: 1) the need for renal replacement therapy, 2) use of antibiotics and positive culture, and 3) inflammatory and oxygenation markers. There was no difference in mortality, need for renal replacement therapy, use of antibiotics or positive cultures between the two groups. The use of corticosteroids was more frequent in the treatment group. Levels of C-reactive protein (CRP) and WBC (white blood cells) counts declined significantly faster in the treatment group. Oxygenation markers rose significantly higher in patients in the tocilizumab group as compared to controls. .
Conclusions The improvement in oxygenation and inflammatory markers with no increase secondary infections or intra-hospital mortality suggests that tocilizumab could be an option for patients with progressive COVID-19 after initiation of systemic corticosteroids.
References
Guaraldi, Meschiari, Cozzi-Lepri, Tocilizumab in patients with severe COVID-19: a retrospective cohort study, Lancet Rheumatol
Horby, Lim, Emberson, Mafham, Bell et al., Effect of Dexamethasone in Hospitalized Patients with COVID-19
Kewan, Covut, Mj, Rose, Gopalakrishna et al., Tocilizumab for treatment of patients with severe COVID-19: A retrospective cohort study
Klopfenstein, Zayet, Lohse, Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients, Med Mal Infect
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?, J Autoimmun
Sun, Wang, Cai, Cytokine storm intervention in the early stages of COVID-19 pneumonia, Cytokine Growth Factor Rev
Toniati, Piva, Cattalini, Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy, Autoimmun Rev
Wang, Liu, Wu, The Definition and Risks of Cytokine Release Syndrome in 11 COVID-19-Affected Critically Ill Patients with Pneumonia: Analysis of Disease Characteristics, J Infect Dis
Who, Coronavirus disease (COVID-19): Situation Report -168
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci U S A
DOI record:
{
"DOI": "10.1101/2020.07.13.20149328",
"URL": "http://dx.doi.org/10.1101/2020.07.13.20149328",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Objectives</jats:title><jats:p>Critically ill patients with COVID-19 may suffer from a cytokine release syndrome (CRS) characterized by remarkably high levels of interleukin 6 (IL-6). We assessed the effects of tocilizumab, an IL-6 receptor antagonist, on intra-hospital mortality and development of positive cultures in patients with COVID-19 admitted to the ICU.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Patients with COVID 19 admitted in the ICU who were treated with tocilizumab plus standard care were enrolled and compared to controls.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>COVID-19 severe disease</jats:p></jats:sec><jats:sec><jats:title>Patients</jats:title><jats:p>Patients with severe COVID-19 disease admitted in the ICU.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Tocilizumab 400 mg IV two doses. Standard and intensive medical care as per institutional clinical protocol.</jats:p></jats:sec><jats:sec><jats:title>Measures and Main Results</jats:title><jats:p>Main outcome: 1) intra-hospital mortality; Secondary Outcomes: 1) the need for renal replacement therapy, 2) use of antibiotics and positive culture, and 3) inflammatory and oxygenation markers. There was no difference in mortality, need for renal replacement therapy, use of antibiotics or positive cultures between the two groups. The use of corticosteroids was more frequent in the treatment group. Levels of C-reactive protein (CRP) and WBC (white blood cells) counts declined significantly faster in the treatment group. Oxygenation markers rose significantly higher in patients in the tocilizumab group as compared to controls.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Tocilizumab was associated with rapid improvement in oxygenation and a faster decrease of CRP and WBC counts in patients with COVID-19 and should be evaluated as rescue therapy for patients with progressive disease</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
7,
15
]
]
},
"author": [
{
"affiliation": [],
"family": "Carvalho",
"given": "Victor",
"sequence": "first"
},
{
"affiliation": [],
"family": "Turon",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonçalves",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ceotto",
"given": "Victor Fraga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurtz",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Righy",
"given": "Cássia",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
15
]
],
"date-time": "2020-07-15T17:45:38Z",
"timestamp": 1594835138000
},
"deposited": {
"date-parts": [
[
2021,
1,
12
]
],
"date-time": "2021-01-12T23:45:26Z",
"timestamp": 1610495126000
},
"group-title": "Intensive Care and Critical Care Medicine",
"indexed": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T00:51:23Z",
"timestamp": 1721263883676
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 10,
"issued": {
"date-parts": [
[
2020,
7,
15
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.07.13.20149328",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
7,
15
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
7,
15
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021011105050527000_2020.07.13.20149328v1.1",
"unstructured": "WHO. Coronavirus disease (COVID-19): Situation Report - 168. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. accessed July 6, 2020."
},
{
"DOI": "10.1016/j.cytogfr.2020.04.002",
"doi-asserted-by": "publisher",
"key": "2021011105050527000_2020.07.13.20149328v1.2"
},
{
"DOI": "10.1093/infdis/jiaa387",
"doi-asserted-by": "crossref",
"key": "2021011105050527000_2020.07.13.20149328v1.3",
"unstructured": "Wang W , Liu X , Wu S , et al. The Definition and Risks of Cytokine Release Syndrome in 11 COVID-19-Affected Critically Ill Patients with Pneumonia: Analysis of Disease Characteristics [published Online ahead of print, 2020 Jun 30]. J Infect Dis. 2020; jiaa387."
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"doi-asserted-by": "publisher",
"key": "2021011105050527000_2020.07.13.20149328v1.4"
},
{
"DOI": "10.1073/pnas.2005615117",
"doi-asserted-by": "publisher",
"key": "2021011105050527000_2020.07.13.20149328v1.5"
},
{
"key": "2021011105050527000_2020.07.13.20149328v1.6",
"unstructured": "Guaraldi G , Meschiari M , Cozzi-Lepri A , et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study [published Online ahead of print, 2020 Jun 24]. Lancet Rheumatol. 2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"doi-asserted-by": "publisher",
"key": "2021011105050527000_2020.07.13.20149328v1.7"
},
{
"key": "2021011105050527000_2020.07.13.20149328v1.8",
"unstructured": "Klopfenstein T , Zayet S , Lohse A , et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients [published Online ahead of print, 2020 May 6]. Med Mal Infect. 2020; S0399-077X(20)30129-3"
},
{
"DOI": "10.1016/j.eclinm.2020.100418",
"doi-asserted-by": "crossref",
"key": "2021011105050527000_2020.07.13.20149328v1.9",
"unstructured": "Kewan T , Covut F , Al–Jaghbeer MJ , Rose L , Gopalakrishna KV , Akbik B. Tocilizumab for treatment of patients with severe COVID–19: A retrospective cohort study [published Online ahead of print, 2020 Jun 20]. EClinicalMedicine. 2020; 100418."
},
{
"DOI": "10.1101/2020.06.22.20137273",
"doi-asserted-by": "crossref",
"key": "2021011105050527000_2020.07.13.20149328v1.10",
"unstructured": "Horby P , Lim W , Emberson J , Mafham M , Bell J , Linsell L , et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19: Preliminary Report. medRxiv Preprint 2020. [Epub Ahead of Print]"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.07.13.20149328"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Effects of Tocilizumab in Critically Ill Patients With COVID-19: A Quasi-Experimental Study",
"type": "posted-content"
}