An integrative approach to clinical recovery for COVID-19 patients using an Ayurvedic formulation: A multicentric double-blind randomized control trial
et al., Research Square, doi:10.21203/rs.3.rs-1165680/v1, CTRI/2021/05/033790, Dec 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small RCT with 39 patients treated with NOQ19 and 37 placebo patients, showing improved recovery, without statistical significance. NOQ19 has multiple ingredients including curcumin, andrographis, and antiandrogen glycyrrhiza glabra.
This study is excluded in meta-analysis:
combined treatments may contribute more to the effect seen.
Study covers curcumin and andrographolide.
|
risk of no hospital discharge, 36.8% lower, RR 0.63, p = 0.67, treatment 2 of 39 (5.1%), control 3 of 37 (8.1%), NNT 34, day 7.
|
|
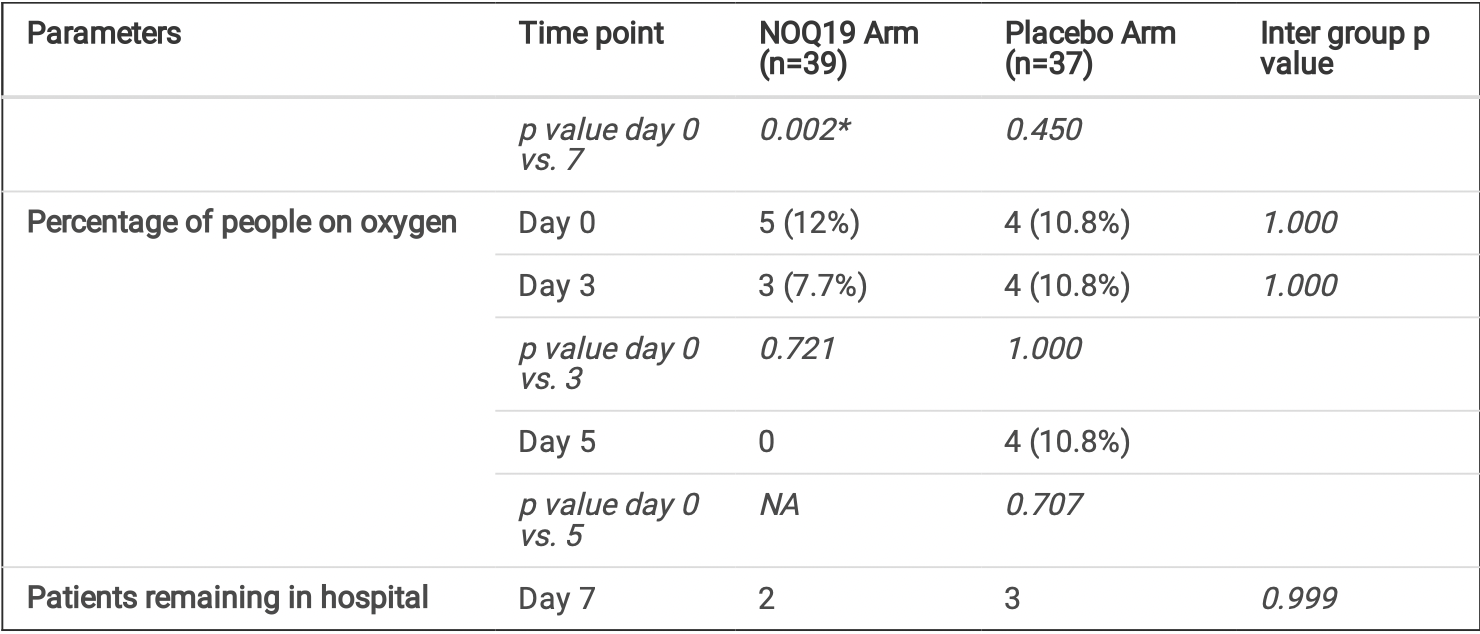
risk of no recovery, 89.1% lower, RR 0.11, p = 0.05, treatment 0 of 39 (0.0%), control 4 of 37 (10.8%), NNT 9.2, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), oxygen supplementation, day 5.
|
|
risk of no recovery, 80.4% lower, RR 0.20, p = 0.23, treatment 0 of 39 (0.0%), control 2 of 37 (5.4%), NNT 18, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 7, dsypnea.
|
|
risk of no recovery, 2.8% higher, RR 1.03, p = 1.00, treatment 13 of 39 (33.3%), control 12 of 37 (32.4%), day 7, fever.
|
|
risk of no viral clearance, 24.1% lower, RR 0.76, p = 0.47, treatment 12 of 39 (30.8%), control 15 of 37 (40.5%), NNT 10, day 7.
|
|
risk of no viral clearance, 5.1% lower, RR 0.95, p = 1.00, treatment 19 of 39 (48.7%), control 19 of 37 (51.4%), NNT 38, day 5.
|
|
risk of no viral clearance, 12.4% lower, RR 0.88, p = 0.47, treatment 24 of 39 (61.5%), control 26 of 37 (70.3%), NNT 11, day 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bhardwaja et al., 27 Dec 2021, Double Blind Randomized Controlled Trial, India, preprint, 26 authors, study period 7 June, 2021 - 28 August, 2021, this trial uses multiple treatments in the treatment arm (combined with multiple treatments) - results of individual treatments may vary, trial CTRI/2021/05/033790.
An integrative approach to clinical recovery for COVID-19 patients using an Ayurvedic formulation: A multicentric double-blind randomized control trial.
doi:10.21203/rs.3.rs-1165680/v1
Background Traditional medicine systems such as Ayurveda contain a vast repository of naturally occurring herbs with strong antimicrobial potency. A multitude of complementary therapies have been explored for their therapeutic role to treat COVID-19 during the pandemic. There have been promising results reported from in silico, in vitro and in vivo studies that need to be further explored in humans.
Objective The present randomized placebo control trial evaluates the clinical e cacy of an integrated approach including Ayurvedic polyherbal formulation, NOQ19 in the improvement of mild to moderate category of COVID-19 infected patients.
Patients and methods A multicentric, randomized, placebo control design was adopted for the study. A total of 76 patients with positive COVID-19 RT-PCR test were enrolled in the study; 39 in the NOQ19 arm and 37 in the placebo arm. Patients were randomized and blinded to their respective intervention, which was provided along with the standard of care treatment. Rate of recovery assessment was done on Day 3, 5 and 7 via RT-PCR test to measure the rate of recovery. Blood markers were analysed on Day 0 and Day 7.
Outcomes The patients were assessed for rate of recovery via RT-PCR and improvement in blood biomarkers. They were also monitored for any adverse events or side effects of the drug.
Results The present study demonstrated a signi cant early recovery in the patients who took the NOQ19 formulation. Patients who received NOQ19 with the standard care of treatment showed clinical improvement in terms of oxygen requirement, breathlessness and SpO2, though the difference was not statistically signi cant. Moreover, no side effects were observed with the use of NOQ19.
Conclusion An overall integrated approach of standard of care treatment with Ayurvedic formulation (NOQ19) results in early clinical outcome in the management of mild to moderate cases of COVID-19.
Previous studies on the same formulation NOQ19 have shown promising results with respect to rate of recovery and antiviral e cacy [23] [24] [25] . A review of Ayurvedic literature presents robust preclinical evidence for e cacy of multiple NOQ19 ingredients such as Ashwagandha (Withania somnifera) and Guduchi (Tinospora cordifolia), along with Amalaki (Phyllanthus emblica) in proliferation of B and T cells and activation of nonspeci c immunity 32 . In addition, several other components of NOQ19 have been highlighted for their antiviral properties previously 8, 33, 34 . Yashtimadhu (Glycyrrhiza glabra) contains glycyrrhizin, a strong antiviral compound. In a previous study, treatment with different concentrations of glycyrrhizin lowered SARS-CoV viral antigen in a cell culture. At a concentration of 4000mg/ ml, Glycyrrhizin completely blocked the viral replication 35 . A key observation of our study was the improvement in oxygen levels and reduction in breathlessness among patients with COVID-19, when treated with NOQ19. An earlier study demonstrated the therapeutic e cacy of Vasaka (Adhatoda vasica), present in NOQ19, in an in vitro and in vivo model, via inhibiting the hypoxic response in both the models. A possible mechanism for this could be the reversal of mitochondrial dysfunction associated with hypoxic conditions like asthma, ARDS, etc. 36 . Presence of Vasaka (Adhatoda Vasica) in NOQ19 may be responsible for its antihypoxic effect on COVID-19 patients 37 ...
References
Anagha, Manasi, Priya, Scope of Glycyrrhiza glabra (Yashtimadhu) as an antiviral agent: a review, Int J Curr Microbio. Ap. Sci
Bajpai, Wadhwa, COVID-19 in India: Disease Burden, Managing the second wave and Innovations
Bogam, Joshi, Nagarkar, The burden of COVID-19 and Case Fatality Rate in Pune India: An Analysis of First and Second Wave of the Pandemic, medRxiv
Chopra, Gautam, Tillu, immunogenicity, and protection with COVID-19 vaccine: A Study Protocol
Cinatl, Morgenstern, Bauer, Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus, Lancet
Gheware, Panda, Khanna, Adhatoda vasica rescues the hypoxia-dependent severe asthma symptoms and mitochondrial dysfunction, J. Physiol. Lung Cell Mol. Physiol
Gupta, Prajapati, A clinical review of different formulations of Vasa (Adhatoda vasica) on Tamaka Shwasa (asthma)
Hanff, Mohareb, Giri, Thrombosis in COVID-19, Am J Hematol
Hoever, Baltina, Michaelis, Antiviral activity of glycyrrhizic acid derivatives against SARS− coronavirus, J Med Chem
Israel, Determining sample size
Joshi, Kamath, Nair, Modulation of neutrophil (dys) function by Ayurvedic herbs and its potential in uence on SARS-CoV-2 infection, J Ayurveda Integr Med
Kanchibhotla, Harsora, Subramanian, Venkatesh, E cacy of a polyherbal formulation in the treatment of SARS CoV-2 disease: An open labelled feasibility study, doi:10.21203/rs.3.rs-923003/v1
Kanchibhotla, Subramanian, Hv, To study the in-vivo e cacy and safety of AYUSH polyherbal formulation among COVID-19 infected Syrian gold hamsters, Researchsquare, doi:10.21203/rs.3.rs-936462/v1
Kanchibhotla, Subramanian, Reddy, An In-vitro evaluation of a polyherbal formulation, against SARS-CoV-2, doi:10.21203/rs.3.rs-936472/v1
Kataky, Handique, A brief overview on Andrographis paniculata (Burm. f) Nees., a high valued medicinal plant: Boon over synthetic drugs, Asian J Sci Technol
Kotecha, The journey with COVID-19: initiatives by Ministry of AYUSH, J Ayurveda Integr Med
Krutika, Experimental and Clinical Evidence of Andrographis paniculata (Roxb
Kumar, Mukherjee, Sharma, Clinical pro le of hospitalized COVID-19 patients in rst and second wave of the pandemic: insights from an Indian registry based observational study, Indian J Med Res
Malabadi, Kolkar, Meti, TRADITIONAL HERBAL FOLK MEDICINE USED FOR CONTROLLING CORONA VIRUS (SARS-COV-2) DISEASE (COVID-19), Int j of innov and sci res
Malabadi, Meti, Chalannavar, Role of herbal medicine for controlling coronavirus (SARS-CoV-2) disease (COVID-19), International Journal of Research and Scienti c Innovations
Maurya, Evaluation of Yashtimadhu (Glycyrrhiza glabra) active phytochemicals against novel coronavirus (SARS-CoV-2
Nivetha, Bhuvaragavan, Kumar, Inhibition of multiple SARS-CoV-2 proteins by an antiviral biomolecule, seselin from Aegle marmelos deciphered using molecular docking analysis, J Biomol Struct Dyn
Patwardhan, Sarwal, Signi cance of AYUSH: India's rst line of defence against COVID-19, J Ayurveda Integr Med
Qazi, Das, Khuntia, In Silico Molecular Docking and Molecular Dynamic Simulation Analysis of Phytochemicals From Indian Foods as Potential Inhibitors of SARS-CoV-2 RdRp and 3CLpro, Nat Prod Commun
Ram, Munikumar, Raju, In silico evaluation of the compounds of the ayurvedic drug, AYUSH-64, for the action against the SARS-CoV-2 main protease, J Ayurveda Integr Med
Rizvi, Tripathy, Sharma, Effect of prophylactic use of intra-nasal oil formulations in the hamster model of Covid-19, bioRxiv
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Shree, Mishra, Selvaraj, Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants-Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)-a molecular docking study, J Biomol Struct Dyn
Singh, Goel, Bourgeade, Ayurveda Rasayana as antivirals and immunomodulators: potential applications in COVID-19
Singh, Goel, Bourgeade, Ayurveda Rasayana as antivirals and immunomodulators: potential applications in COVID-19, EPSR
Singh, Goel, Bourgeade, Ayurveda Rasayana as antivirals and immunomodulators: potential applications in COVID-19, ESPR
Soni, Mehta, Ratre, Curcumin, a traditional spice component, can hold the promise against COVID-19?, Eur J Pharmacol
Subhrajyoti, Immunomodulatory herbs of Ayurveda and covid-19: a review article, Journal of Ayurveda and Integrated Medical Sciences
Tripathi, Singh, Sharma, Identi cation of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor, J Biomol Struct Dyn
Vetrivel, Deshpande, Hegde, Phytochemical moieties from Indian traditional medicine for targeting dual hotspots on SARS-CoV-2 spike protein: an integrative in-silico approach, Front Med
Vincent, Arokiyaraj, Saravanan, Molecular docking studies on the anti-viral effects of compounds from Kabasura Kudineer on SARS-CoV-2 3CLpro, Front Mol Biosci
Wanjarkhedkar, Sarade, Purandare, A prospective clinical study of an Ayurveda regimen in COVID 19 patients, J Ayurveda Integr Med
Willcox, Bodeker, Traditional herbal medicines for malaria, Bmj
Zahedipour, Hosseini, Sathyapalan, Potential effects of curcumin in the treatment of COVID-19 infection, Phytother Res
DOI record:
{
"DOI": "10.21203/rs.3.rs-1165680/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-1165680/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p><jats:bold>Background</jats:bold>Traditional medicine systems such as Ayurveda contain a vast repository of naturally occurring herbs with strong antimicrobial potency. A multitude of complementary therapies have been explored for their therapeutic role to treat COVID-19 during the pandemic. There have been promising results reported from <jats:italic>in silico</jats:italic>, <jats:italic>in vitro</jats:italic> and <jats:italic>in vivo</jats:italic> studies that need to be further explored in humans.<jats:bold>Objective</jats:bold>The present randomized placebo control trial evaluates the clinical efficacy of an integrated approach including Ayurvedic polyherbal formulation, NOQ19 in the improvement of mild to moderate category of COVID-19 infected patients.<jats:bold>Patients and methods</jats:bold>A multicentric, randomized, placebo control design was adopted for the study. A total of 76 patients with positive COVID-19 RT-PCR test were enrolled in the study; 39 in the NOQ19 arm and 37 in the placebo arm. Patients were randomized and blinded to their respective intervention, which was provided along with the standard of care treatment. Rate of recovery assessment was done on Day 3, 5 and 7 via RT-PCR test to measure the rate of recovery. Blood markers were analysed on Day 0 and Day 7.<jats:bold>Outcomes</jats:bold>The patients were assessed for rate of recovery via RT-PCR and improvement in blood biomarkers. They were also monitored for any adverse events or side effects of the drug.<jats:bold>Results</jats:bold>The present study demonstrated a significant early recovery in the patients who took the NOQ19 formulation. Patients who received NOQ19 with the standard care of treatment showed clinical improvement in terms of oxygen requirement, breathlessness and SpO2, though the difference was not statistically significant. Moreover, no side effects were observed with the use of NOQ19.<jats:bold>Conclusion</jats:bold>An overall integrated approach of standard of care treatment with Ayurvedic formulation (NOQ19) results in early clinical outcome in the management of mild to moderate cases of COVID-19.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
12,
13
]
]
},
"author": [
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Bhardwaja",
"given": "Pankaj",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Sri Manakula Vinayagar Medical College and Hospital"
}
],
"family": "Ganapathy",
"given": "Kalaiselvan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Rishikesh"
}
],
"family": "Pathania",
"given": "Monika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Naveen",
"given": "K H",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Charan",
"given": "Jaykaran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Dutta",
"given": "Siddhartha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Gadepalli",
"given": "Ravisekhar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Sriniva",
"given": "Srikanth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Gupta",
"given": "Manoj Kumar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Goelj",
"given": "Akhil D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Midha",
"given": "Naresh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Kumar",
"given": "Bharat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Sharma",
"given": "Meenakshi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Sharma",
"given": "Praveen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Banerjee",
"given": "Mithu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Mitra",
"given": "Prasenjit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Jodhpur"
}
],
"family": "Misra",
"given": "Sanjeev",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sri Manakula Vinayagar Medical College and Hospital"
}
],
"family": "V.",
"given": "Vinayagamoorthy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sri Manakula Vinayagar Medical College and Hospital"
}
],
"family": "Subramaniant",
"given": "Girija",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sri Manakula Vinayagar Medical College and Hospital"
}
],
"family": "R",
"given": "Praveen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Rishikesh"
}
],
"family": "Dhar",
"given": "Minakshi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Rishikesh"
}
],
"family": "Saxena",
"given": "Vartika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Rishikeh"
}
],
"family": "Dhamija",
"given": "Puneet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AIIMS Rishikesh"
}
],
"family": "Singh",
"given": "Archana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "SRI SRI INSTITUTE FOR ADVANCED RESEARCH"
}
],
"family": "Subramanian",
"given": "Saumya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "SRI SRI INSTITUTE FOR ADVANCED RESEARCH"
}
],
"family": "Kanchibhotlaz",
"given": "Divya",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
28
]
],
"date-time": "2021-12-28T00:04:15Z",
"timestamp": 1640649855000
},
"deposited": {
"date-parts": [
[
2021,
12,
28
]
],
"date-time": "2021-12-28T00:04:26Z",
"timestamp": 1640649866000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T06:39:00Z",
"timestamp": 1648881540170
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
12,
27
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
27
]
],
"date-time": "2021-12-27T00:00:00Z",
"timestamp": 1640563200000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-1165680/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-1165680/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
12,
27
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2021,
12,
27
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-1165680/v1"
}
},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"An integrative approach to clinical recovery for COVID-19 patients using an Ayurvedic formulation: A multicentric double-blind randomized control trial."
],
"type": "posted-content"
}
bhardwaja
