Clinical Features, Pathobiology, Efficacy, and Toxicity of Tenofovir Disoproxil Fumarate and Emtricitabine for Mild to Moderate SARS-CoV-2 Infections
et al., European Journal of Respiratory Medicine, doi:10.31488/EJRM.122, NCT04712357, Dec 2021
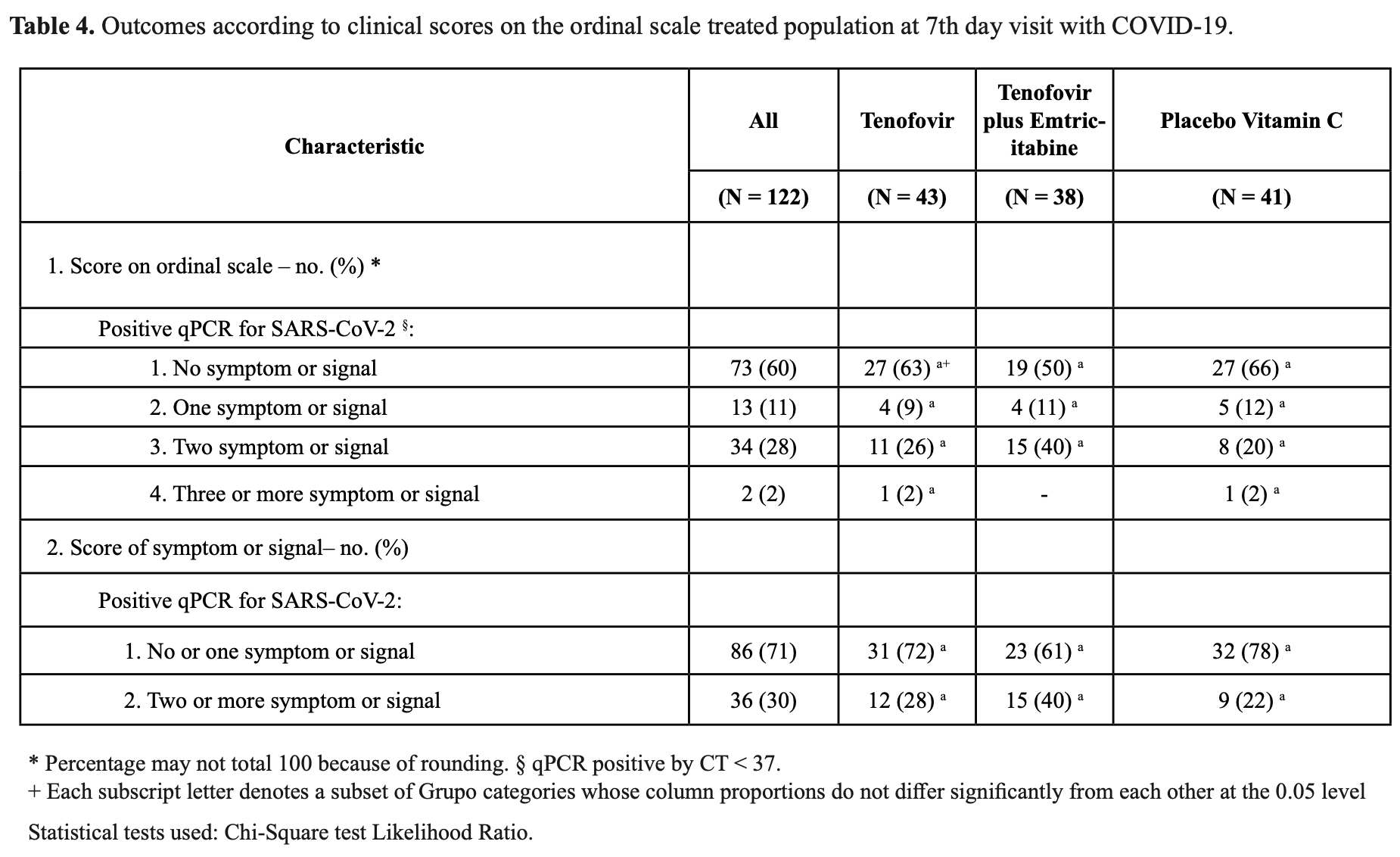
RCT 226 outpatients showing no significant difference with tenofovir disoproxil fumarate (TDF) alone or combined with emtricitabine (FTC) for mild to moderate COVID-19, compared with vitamin C.
|
risk of hospitalization, 80.4% lower, RR 0.20, p = 0.24, treatment 0 of 43 (0.0%), control 2 of 41 (4.9%), NNT 20, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 9.0% higher, RR 1.09, p = 0.82, treatment 16 of 43 (37.2%), control 14 of 41 (34.1%), day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Arruda et al., 31 Dec 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, 19 authors, study period 9 November, 2020 - 5 July, 2021, trial NCT04712357 (history).
Contact: alima@ufc.br.
Clinical Features, Pathobiology, Efficacy, and Toxicity of Tenofovir Disoproxil Fumarate and Emtricitabine for Mild to Moderate SARS-CoV-2 Infections
European Journal of Respiratory Medicine, doi:10.31488/ejrm.122
This study evaluated the efficacy of tenofovir disoproxil fumarate (TDF) and TDF combined with emtricitabine (FTC) in patients with COVID-19 infections (ClinicalTrials.gov #NCT04712357). We conducted a randomized, double-blind, placebo-controlled clinical trial in patients with mild to moderate respiratory infection caused by SARS-CoV-2. Patients were randomly recruited to take 10 days of TDF (300 mg/day), TDF (300 mg/day) combined with FTC (200 mg/day) or placebo vitamin C (500 mg/day). From a total of 309 patients with clinical suspicion of SARS-CoV-2, 227 met the inclusion criteria and were randomly distributed into the following groups: (a) 75 (one did not initiate treatment) in the TDF; (b) 74 in the TDF combined with FTC; and (c) 77 in the vitamin C (placebo). Fever (≥37.8°C), ageusia or dysgeusia, anosmia or dysosmia, and two or more clinical signs and symptoms were significantly associated with SARS-CoV-2 infection. There was no significant change in clinical score based on clinical signs and symptoms between treatment groups. Patients with infection by SARS-CoV-2 had higher concentrations of G-CSF, IL-1β, IL-6 and TNF-α compared to patients without infection. In conclusions, patientswith fever (≥37.8°C), ageusia or dysgeusia, anosmia or dysosmia, and two or more signs and symptoms, had a better prediction for the diagnosis of COVID-19. In conclusions, patients with SARS-CoV-2 showed higher and more persistent proinflammatory cytokines and chemokines profile compared to patients not infected with SARS-CoV-2. Pharmacological intervention with TDF or TDF combined with FTC did not change the clinical signs and symptoms score assessed on the seventh day in patients with SARS-CoV-2. A graphical abstract is shown in Figure 1 .
Author Contributions
References
Abdo, Elfiky, SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp) Targeting: An in silico, Perspective. J Biomol Struct Dyn
Amo, Polo, Moreno, Antiretrovirals and Risk of COVID-19 Diagnosis and Hospitalization in HIV-Positive Persons, Epidemiol
Amo, Polo, Moreno, Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy, Ann Intern Med
Andrei, Gillemot, Topalis, The Anti-Human Immunodeficiency Virus Drug Tenofovir, a Reverse Transcriptase Inhibitor, Also Targets the Herpes Simplex Virus DNA Polymerase, J Infect Dis
Beigel, Tomashek, Dodd, for the ACTT-1 Study Group Members.Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Buchbinder, Liu, CROI 2019: Advances in HIV prevention and plans to end the epidemic, Top. Antivir. Med
Celum, Hong, Cent, Herpes Simplex Virus Type 2 Acquisition among HIV-1-Infected Adults Treated with Tenofovir Disoproxyl Fumarate as Part of Combination Antiretroviral Therapy: Results from the ACTG A5175 PEARLS Study, J Infect Dis
Chaix, Charreau, Pintado, Effect of On-Demand Oral Pre-exposure Prophylaxis with Tenofovir/Emtricitabine on Herpes Simplex Virus-1/2 Incidence among Men Who Have Sex with Men: A Substudy of the ANRS IPERGAY Trial, Open Forum. Infect Dis
Copertino, Lima, Duarte, Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection, J Biomol Struct Dyn
Dallocchio, Dessì, Vito, Early combination treatment with existing HIV antivirals: An effective treatment for COVID-19?, Eur Rev Med Pharmacol Sci
Dejong, Spinelli, Okochi, Gandhi, Tenofovir-based PrEP for COVID-19: an untapped opportunity?, AIDS
Elfiky, Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study, Life Sci
Feng, Chen, Xue, MCCS: A novel recognition pattern-based method for fast track discovery of anti-SARS-CoV-2 drugs, Brief Bioinform
Filho, Junior, Lima, Perinatal Outcomes of Asynchronous Influenza Vaccination, Ceará, Brazil, 2013-2018, Emerg Infect Dis
Giuliano C Clososki, Soldi, Silva, Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2, J Braz Chem Soc
Golob, Lugogo, Lauring, SARS-CoV-2 vaccines: A triumph of science and collaboration, JCI Insight
Gordon, Tchesnokov, Woolner, Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J Biol Chem
Grahl, Alcará, Souza, Evaluation of drug repositioning by molecular docking of pharmaceutical resources available in the Brazilian healthcare system against SARS-CoV-2, Inform Med Unlocked
Hasan, Kamruzzaman, Manjur, Structural analogues of existing anti-viral drugs inhibit SARS-CoV-2 RNA dependent RNA polymerase: A computational hierarchical investigation, Heliyon
Hou, Okuda, Edwards, SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract, Cell
Jeong, Song, Yoon, Therapeutic Strategies against COVID-19 and Structural Characterization of SARS-CoV-2: A Review, Front. Microbiol
Karaba, Zhou, Hsieh, Differential Cytokine Signatures of SARS-CoV-2 and Influenza Infection Highlight Key Differences in Pathobiology, Clin Infect Dis
Kuba, Imai, Rao, A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat Med
Kumar, Dowling, Román, Status Report on COVID-19 Vaccines Development, Curr Infect Dis Rep
Long, Hall, Cunningham, Influenza surveillance in community-dwelling elderly compared with children, Arch Fam Med
Maria, Barillari, Bastiani, Jerome R Lechien, A structural equation model to examine the clinical features of mild-to-moderate COVID-19: A multicenter Italian study, J Med Virol
Marrazzo, Rabe, Kelly, Tenofovir Gel for Prevention of Herpes Simplex Virus Type 2 Acquisition: Findings from the VOICE Trial, J Infect Dis
Monto, Gravenstein, Elliott, Clinical signs and symptoms predicting influenza infection, Arch Intern Med
Mu, Pham, Podany, Evaluating emtricitabine + rilpivirine + tenofovir alafenamide in combination for the treatment of HIV-infection, Expert Opin. Pharmacother
Parienti, Prazuck, Peyro-Saint-Paul, Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial, E Clin Med
Park, Yu, Kin, Kim, Antiviral Efficacies of FDA-Approved Drugs against SARS-CoV-2 Infection in Ferrets, mBio
Pormohammad, Ghorbani, Khatami, Comparison of influenza type A and B with COVID-19: A global systematic review and meta-analysis on clinical, laboratory and radiographic findings, Rev Med Virol
Poustforoosh, Hashemipour, Tüzün, Evaluation of potential anti-RNA-dependent RNA polymerase (RdRP) drugs against the newly emerged model of COVID-19 RdRP using computational methods, Biophys Chem
Raberahona, Rakotomalala, Rakotomijoro, Clinical and epidemiological features discriminating confirmed COVID-19 patients from SARS-CoV-2 negative patients at screening centres in Madagascar, Int J Infect Dis
Rahman, Banik, Chowdhury, Identification of potential antivirals against SARS-CoV-2 using virtual screening method, Inform. Med Unlocked
Salpini, Alkhatib, Costa, Key genetic elements, single and in clusters, underlying geographically dependent SARS-CoV-2 genetic adaptation and their impact on binding affinity for drugs and immune control, J Antimicrob Chemother
Santevecchi, Miller, Childs-Kean, Doing More with Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tab-let Regimen for Antiretroviral-Naïve Adults with HIV-1 Infection, Ann Pharmaco ther
Shah, Modi, Sagar, In silico studies on therapeutic agents for COVID-19: Drug repurposing approach, Life Sci
Song, Hu, Yu, Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages, Cytometry A
Su, Wang, Yoo, Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2, Sci Rep
Tiwari, Denovo designing, retro-combinatorial synthesis, and molecular dynamics analysis identify novel antiviral VTRM1.1 against RNA-dependent RNA polymerase of SARS CoV2 virus, Int J Biol Macromol
Toor, Banerjee, Rath, Computational drug re-purposing targeting the spike glycoprotein of SARS-CoV-2 as an effective strategy to neutralize COVID-19, Eur J Pharmacol
Udofia, Gbayo, Oa, In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV, Netw Model Anal Health Inform Bioinform
Uyeki, Influenza, None, Ann Intern Med
Waters, Mehta, Gogtay, The evidence for using tenofovir disoproxil fumarate plus lamivudine as a nucleoside analogue backbone for the treatment of HIV, J Virus Erad
Wrapp, Wang, Corbett, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science
Yun, Song, Ji, Identification of therapeutic drugs against COVID-19 through computational investigation on drug repurposing and structural modification, J Biomed Res
Zanella, Zizioli, Castelli, Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection, Pharmaceuticals
DOI record:
{
"DOI": "10.31488/ejrm.122",
"ISSN": [
"2633-7452"
],
"URL": "http://dx.doi.org/10.31488/EJRM.122",
"container-title": "European Journal of Respiratory Medicine",
"container-title-short": "Eur J Respir Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
21
]
],
"date-time": "2022-11-21T19:43:17Z",
"timestamp": 1669059797000
},
"deposited": {
"date-parts": [
[
2022,
11,
21
]
],
"date-time": "2022-11-21T19:43:18Z",
"timestamp": 1669059798000
},
"indexed": {
"date-parts": [
[
2022,
11,
22
]
],
"date-time": "2022-11-22T06:02:08Z",
"timestamp": 1669096928917
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2021
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2021
]
]
}
},
"member": "16388",
"original-title": [],
"prefix": "10.31488",
"published": {
"date-parts": [
[
2021
]
]
},
"published-online": {
"date-parts": [
[
2021
]
]
},
"publisher": "MAK Periodical Library",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://europeanjournalofrespiratorymedicine.com/clinical-features-pathobiology-efficacy-and-toxicity-of-tenofovir-disoproxil-fumarate-and-emtricitabine-for-mild-to-moderate-sars-coV-2-infections"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical Features, Pathobiology, Efficacy, and Toxicity of Tenofovir Disoproxil Fumarate and Emtricitabine for Mild to Moderate SARS-CoV-2 Infections",
"type": "journal-article",
"volume": "3"
}