Azvudine reduces the mortality rate of patients with Coronavirus disease 2019: a single-center retrospective analysis study
et al., Research Square, doi:10.21203/rs.3.rs-4157424/v1, Apr 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 2,862 hospitalized COVID-19 patients in China showing lower mortality with azvudine treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
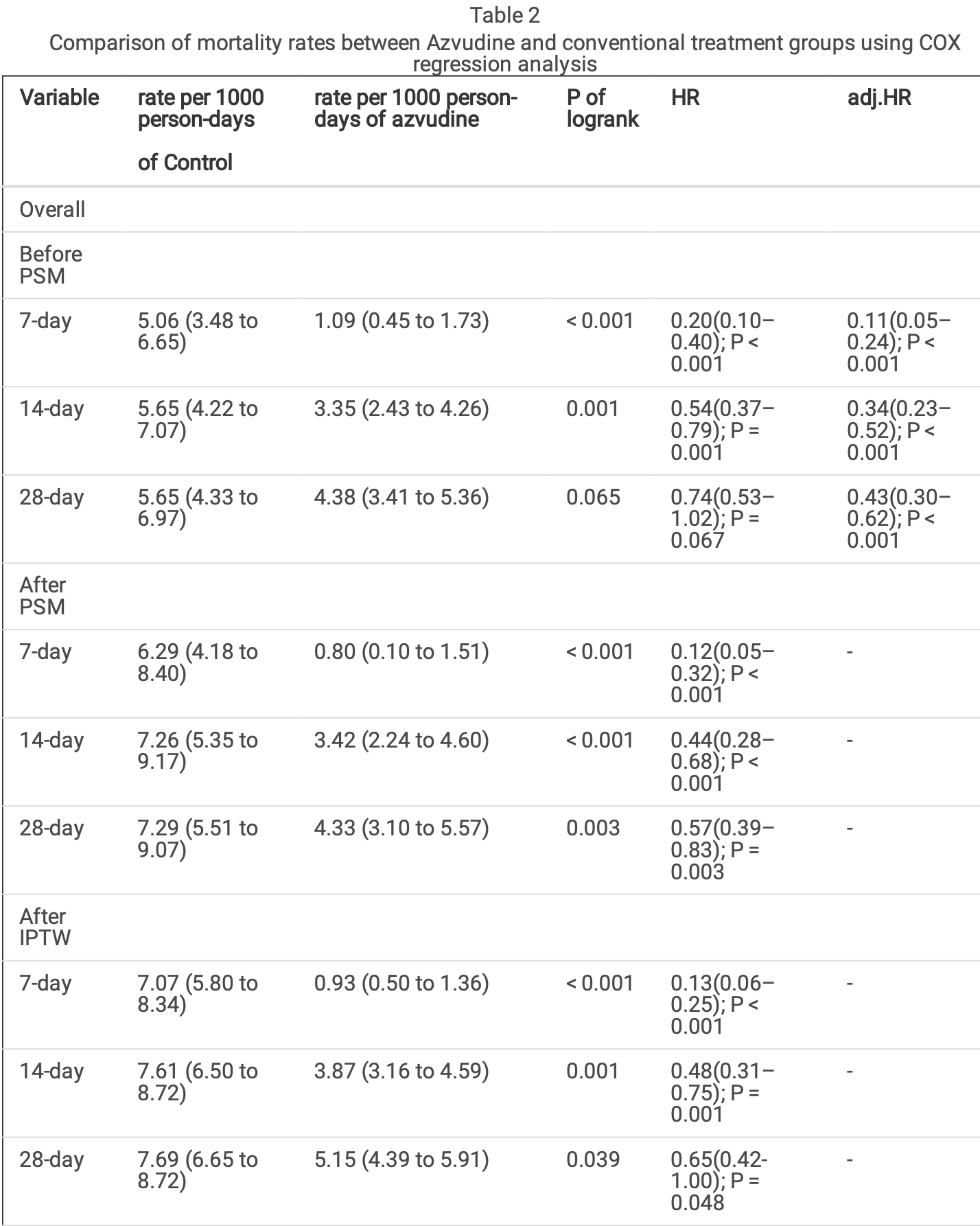
risk of death, 35.0% lower, HR 0.65, p = 0.048, treatment 1,490, control 1,373, propensity score weighting, day 28.
|
|
risk of death, 52.0% lower, HR 0.48, p = 0.001, treatment 1,490, control 1,373, propensity score weighting, day 14.
|
|
risk of death, 87.0% lower, HR 0.13, p = 0.001, treatment 1,490, control 1,373, propensity score weighting, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Zhong et al., 1 Apr 2024, retrospective, China, preprint, 7 authors, study period 1 December, 2022 - 31 March, 2023.
Azvudine reduces the mortality rate of patients with Coronavirus disease 2019: a single-center retrospective analysis study
doi:10.21203/rs.3.rs-4157424/v1
Background: Several therapeutic drugs have been authorized for the treatment of patients with Coronavirus disease 2019 (COVID-19). However, further research on the mechanisms of action, e cacy, and target populations of these novel therapeutic drugs are necessary. Hence, this study aimed to investigate the effectiveness of azvudine in hospitalized patients with COVID-19. Methods: We conducted a retrospective cohort study of patients with COVID-19 admitted to our hospital from December 1, 2022, to March 31, 2023. Patients were divided into retrospective cohorts receiving azvudine antiviral therapy and standard treatment, and were followed-up for up to 28 days. Results: Prior to data processing, azvudine treatment was associated with reduced mortality rates at 7 days (1.09/1000 persons vs.5.06/1000 persons, p<0.001)and 14 days (3.35/1000 persons vs. 5.65/1000 persons, p=0.001). After propensity score matching, a decrease in mortality rates at 7 days (0.08/1000 persons vs.6.29/1000 persons, p<0.001), 14 days (3.42/1000 persons vs. 7.26/1000 persons, p<0.001), and 28 days (4.33/1000 persons vs. 7.29/1000 persons, p=0.003) were observed following azvudine treatment. After inverse probability of treatment weighting adjustment, the results were consistent with propensity score matching. In the clinical subgroup analysis, for hospitalized severe and critical patients with COVID-19, azvudine treatment intervention signi cantly reduced patient mortality rates. Conclusions: The study suggests that in hospitalized patients with COVID-19, azvudine treatment signi cantly reduces patient mortality rates in hospitalized COVID-19 infections, wherein the effects are more pronounced in severe and critical patients.
Declarations Ethics approval and consent to participate was no direct involvement in the conception, design, or implementation of this study. The requirement for patient consent was waived for this retrospective study, which utilized data from electronic medical records. This study was approved by the Ethics Committee of the First A liated Hospital of the Gannan Medical University Hospital (LLSL-2024065).
Consent for publication Not applicable.
Competing interests The authors declare that they have no competing interests.
Author Contributions Statement XianfaL JZ designed the experiments. XiaoL was responsible for clinical assessment of patients. LZ, XZ, LR,and ZZ collected the data. JZ was responsible for data management. JZ and ZZ conducted the statistical analysis. This article was written by ZZ, and reviewed by XianfaL. All the authors have reviewed and approved of the nal manuscript.
Supplementary Files This is a list of supplementary les associated with this preprint. Click to download. azvudineattachment.docx
References
Bertuccio, The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: a retrospective study, Infection, doi:10.1007/s15010-023-02028-5
Deng, Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study, Journal of medical virology, doi:10.1002/jmv.28756
Deng, Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study, Journal of medical virology, doi:10.1002/jmv.28756
Fayzullina, FNC: An Advanced Anticancer Therapeutic or Just an Underdog?, Frontiers in oncology, doi:10.3389/fonc.2022.820647
Gao, Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, The Journal of infection, doi:10.1016/j.jinf.2023.03.023
Gentile, Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study, Vaccines, doi:10.3390/vaccines10101731
Gentile, Scotto, Schiano Moriello, Pinchera, Villari et al., Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a reallife study, Vaccines, doi:10.3390/vaccines10101731
Lui, Analysis of All-Cause Hospitalization and Death Among Nonhospitalized Patients With Type 2 Diabetes and SARS-CoV-2 Infection Treated With Molnupiravir or Nirmatrelvir-Ritonavir During the Omicron Wave in Hong Kong, JAMA network open, doi:10.1001/jamanetworkopen.2023.14393
Morris, Lee, Nirmatrelvir for Nonhospitalized Adults with Covid-19, The New England journal of medicine, doi:10.1056/NEJMc2206277
Shen, Xiao, Sun, Li, Wu et al., Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study, medRxiv, doi:10.1101/2023.01.23.23284899
Wang, Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study, EClinicalMedicine, doi:10.1016/j.eclinm.2024.102468
Wong, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with con rmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Xie, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, BMJ (Clinical research, doi:10.1136/bmj-2022-074572
Yu, Chang, The rst Chinese oral anti-COVID-19 drug Azvudine launched, Innovation, doi:10.1016/j.xinn.2022.100321
Zhang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal transduction and targeted therapy, doi:10.1038/s41392-021-00835-6
Zhou, Azvudine and nirmatrelvir-ritonavir in hospitalized patients with moderate-tosevere COVID-19: Emulation of a randomized target trial, Journal of medical virology, doi:10.1002/jmv.29318
DOI record:
{
"DOI": "10.21203/rs.3.rs-4157424/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-4157424/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p><jats:bold>Background: </jats:bold>Several therapeutic drugs have been authorized for the treatment of patients with Coronavirus disease 2019 (COVID-19). However, further research on the mechanisms of action, efficacy, and target populations of these novel therapeutic drugs are necessary. Hence, this study aimed to investigate the effectiveness of azvudine in hospitalized patients with COVID-19.\n<jats:bold>Methods: </jats:bold>We conducted a retrospective cohort study of patients with COVID-19 admitted to our hospital from December 1, 2022, to March 31, 2023. Patients were divided into retrospective cohorts receiving azvudine antiviral therapy and standard treatment, and were followed-up for up to 28 days.\n<jats:bold>Results:</jats:bold> Prior to data processing, azvudine treatment was associated with reduced mortality rates at 7 days (1.09/1000 persons vs.5.06/1000 persons,<jats:italic> p</jats:italic><0.001)and 14 days (3.35/1000 persons vs. 5.65/1000 persons,<jats:italic> p</jats:italic>=0.001). After propensity score matching, a decrease in mortality rates at 7 days (0.08/1000 persons vs.6.29/1000 persons, <jats:italic>p</jats:italic><0.001), 14 days (3.42/1000 persons vs. 7.26/1000 persons, <jats:italic>p</jats:italic><0.001), and 28 days (4.33/1000 persons vs. 7.29/1000 persons, <jats:italic>p</jats:italic>=0.003) were observed following azvudine treatment. After inverse probability of treatment weighting adjustment, the results were consistent with propensity score matching. In the clinical subgroup analysis, for hospitalized severe and critical patients with COVID-19, azvudine treatment intervention significantly reduced patient mortality rates.\n<jats:bold>Conclusions: </jats:bold>The study suggests that in hospitalized patients with COVID-19, azvudine treatment significantly reduces patient mortality rates in hospitalized COVID-19 infections, wherein the effects are more pronounced in severe and critical patients.</jats:p>",
"accepted": {
"date-parts": [
[
2024,
3,
24
]
]
},
"author": [
{
"affiliation": [
{
"name": "First Affiliated Hospital of Gannan Medical University"
}
],
"family": "Zhong",
"given": "Zhen",
"sequence": "first"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Gannan Medical University"
}
],
"family": "Liu",
"given": "Xiao-feng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Gannan Medical University"
}
],
"family": "Zhou",
"given": "Xiao-zhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gannan Medical University"
}
],
"family": "Zhong",
"given": "Jia-ning",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Gannan Medical University"
}
],
"family": "Zhou",
"given": "Li-cheng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Gannan Medical University"
}
],
"family": "Li",
"given": "Rong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Gannan Medical University"
}
],
"family": "Liu",
"given": "Xian-fa",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
4,
1
]
],
"date-time": "2024-04-01T16:37:57Z",
"timestamp": 1711989477000
},
"deposited": {
"date-parts": [
[
2024,
4,
1
]
],
"date-time": "2024-04-01T16:38:02Z",
"timestamp": 1711989482000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T01:21:38Z",
"timestamp": 1712020898680
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
4,
1
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
1
]
],
"date-time": "2024-04-01T00:00:00Z",
"timestamp": 1711929600000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-4157424/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-4157424/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2024,
4,
1
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2024,
4,
1
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"DOI": "10.1056/NEJMc2206277",
"article-title": "“Nirmatrelvir for Nonhospitalized Adults with Covid-19.”",
"author": "Morris Andrew M",
"doi-asserted-by": "publisher",
"first-page": "474",
"issue": "5",
"journal-title": "The New England journal of medicine",
"key": "ref1",
"unstructured": "Morris, Andrew M, and Todd C Lee. “Nirmatrelvir for Nonhospitalized Adults with Covid-19.” The New England journal of medicine vol. 387,5 (2022): 474–475. doi:10.1056/NEJMc2206277.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.3390/vaccines10101731;",
"author": "Gentile Ivan",
"doi-asserted-by": "publisher",
"key": "ref2",
"unstructured": "Gentile, Ivan et al. “Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study.” Vaccines vol. 10,10 1731. 17 Oct. 2022, doi:10.3390/vaccines10101731;",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"article-title": "“Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study",
"author": "Wong Carlos KH",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet (London, England)",
"key": "ref3",
"unstructured": "Wong, Carlos K H et al. “Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study.” Lancet (London, England) vol. 400,10359 (2022): 1213–1222. doi:10.1016/S0140-6736(22)01586-0;",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2022-074572",
"author": "Xie Yan",
"doi-asserted-by": "publisher",
"key": "ref4",
"unstructured": "Xie, Yan et al. “Molnupiravir and risk of post-acute sequelae of covid-19: cohort study.” BMJ (Clinical research ed.) vol. 381 e074572. 25 Apr. 2023, doi:10.1136/bmj-2022-074572.",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "Zhang Jin-Lan",
"doi-asserted-by": "publisher",
"key": "ref5",
"unstructured": "Zhang, Jin-Lan et al. “Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients.” Signal transduction and targeted therapy vol. 6,1 414. 6 Dec. 2021, doi:10.1038/s41392-021-00835-6.",
"year": "2021"
},
{
"DOI": "10.1007/s15010-023-02028-5",
"article-title": "The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: a retrospective study.” Infection",
"author": "Bertuccio Paola",
"doi-asserted-by": "publisher",
"first-page": "1633",
"key": "ref6",
"unstructured": "Bertuccio, Paola et al. “The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: a retrospective study.” Infection vol. 51,6 (2023): 1633–1644. doi:10.1007/s15010-023-02028-5.",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2023.14393",
"article-title": "“Analysis of All-Cause Hospitalization and Death Among Nonhospitalized Patients With Type 2 Diabetes and SARS-CoV-2 Infection Treated With Molnupiravir or Nirmatrelvir-Ritonavir During the Omicron Wave in Hong Kong.” JAMA network open vol. 6,5 e2314393",
"author": "Lui David TW",
"doi-asserted-by": "publisher",
"issue": "1",
"key": "ref7",
"unstructured": "Lui, David T W et al. “Analysis of All-Cause Hospitalization and Death Among Nonhospitalized Patients With Type 2 Diabetes and SARS-CoV-2 Infection Treated With Molnupiravir or Nirmatrelvir-Ritonavir During the Omicron Wave in Hong Kong.” JAMA network open vol. 6,5 e2314393. 1 May. 2023, doi:10.1001/jamanetworkopen.2023.14393.",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"author": "Gao Yuan",
"doi-asserted-by": "publisher",
"key": "ref8",
"unstructured": "Gao, Yuan et al. “Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19.” The Journal of infection vol. 86,6 (2023): e158-e160. doi:10.1016/j.jinf.2023.03.023",
"year": "2023"
},
{
"DOI": "10.3389/fonc.2022.820647",
"author": "Fayzullina Daria",
"doi-asserted-by": "publisher",
"key": "ref9",
"unstructured": "Fayzullina, Daria et al. “FNC: An Advanced Anticancer Therapeutic or Just an Underdog?.” Frontiers in oncology vol. 12 820647. 10 Feb. 2022, doi:10.3389/fonc.2022.820647",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28756",
"article-title": "“Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study.”",
"author": "Deng Guangtong",
"doi-asserted-by": "publisher",
"first-page": "e28756",
"issue": "4",
"journal-title": "Journal of medical virology",
"key": "ref10",
"unstructured": "Deng, Guangtong et al. “Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study.” Journal of medical virology vol. 95,4 (2023): e28756. doi:10.1002/jmv.28756.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3390/vaccines10101731;",
"article-title": "Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study",
"author": "Gentile I",
"doi-asserted-by": "publisher",
"first-page": "1731",
"issue": "10",
"journal-title": "Vaccines",
"key": "ref11",
"unstructured": "Gentile, I., Scotto, R., Schiano Moriello, N., Pinchera, B., Villari, R., Trucillo, E., et al. (2022). Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study. Vaccines 10 (10), 1731. doi:10.3390/vaccines10101731;",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.xinn",
"author": "Yu Bin",
"doi-asserted-by": "publisher",
"key": "ref12",
"unstructured": "Yu, Bin, and Junbiao Chang. “The first Chinese oral anti-COVID-19 drug Azvudine launched.” Innovation (Cambridge (Mass.)) vol. 3,6 (2022): 100321. doi:10.1016/j.xinn.2022.100321;Deng, Guangtong et al. “Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study.” Journal of medical virology vol. 95,4 (2023): e28756. doi:10.1002/jmv.28756.",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2024.102468",
"article-title": "Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study.” EClinicalMedicine",
"author": "Wang Shuxia",
"doi-asserted-by": "publisher",
"journal-title": "9 Feb",
"key": "ref13",
"unstructured": "Wang, Shuxia et al. “Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study.” EClinicalMedicine vol. 69 102468. 9 Feb. 2024, doi:10.1016/j.eclinm.2024.102468.",
"volume": "69 102468",
"year": "2024"
},
{
"DOI": "10.1002/jmv.29318",
"article-title": "“Azvudine and nirmatrelvir-ritonavir in hospitalized patients with moderate-to-severe COVID-19: Emulation of a randomized target trial",
"author": "Zhou Yiling",
"doi-asserted-by": "publisher",
"first-page": "e29318",
"issue": "12",
"journal-title": "Journal of medical virology",
"key": "ref14",
"unstructured": "Zhou, Yiling et al. “Azvudine and nirmatrelvir-ritonavir in hospitalized patients with moderate-to-severe COVID-19: Emulation of a randomized target trial.” Journal of medical virology vol. 95,12 (2023): e29318. doi:10.1002/jmv.29318.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1101/2023.01.23.23284899",
"article-title": "Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Shen M",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref15",
"unstructured": "Shen, M., Xiao, C., Sun, Y., Li, D., Wu, P., Jin, L., et al. (2023). Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study. medRxiv. doi:10.1101/2023.01.23.23284899.",
"year": "2023"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-4157424/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Azvudine reduces the mortality rate of patients with Coronavirus disease 2019: a single-center retrospective analysis study",
"type": "posted-content"
}