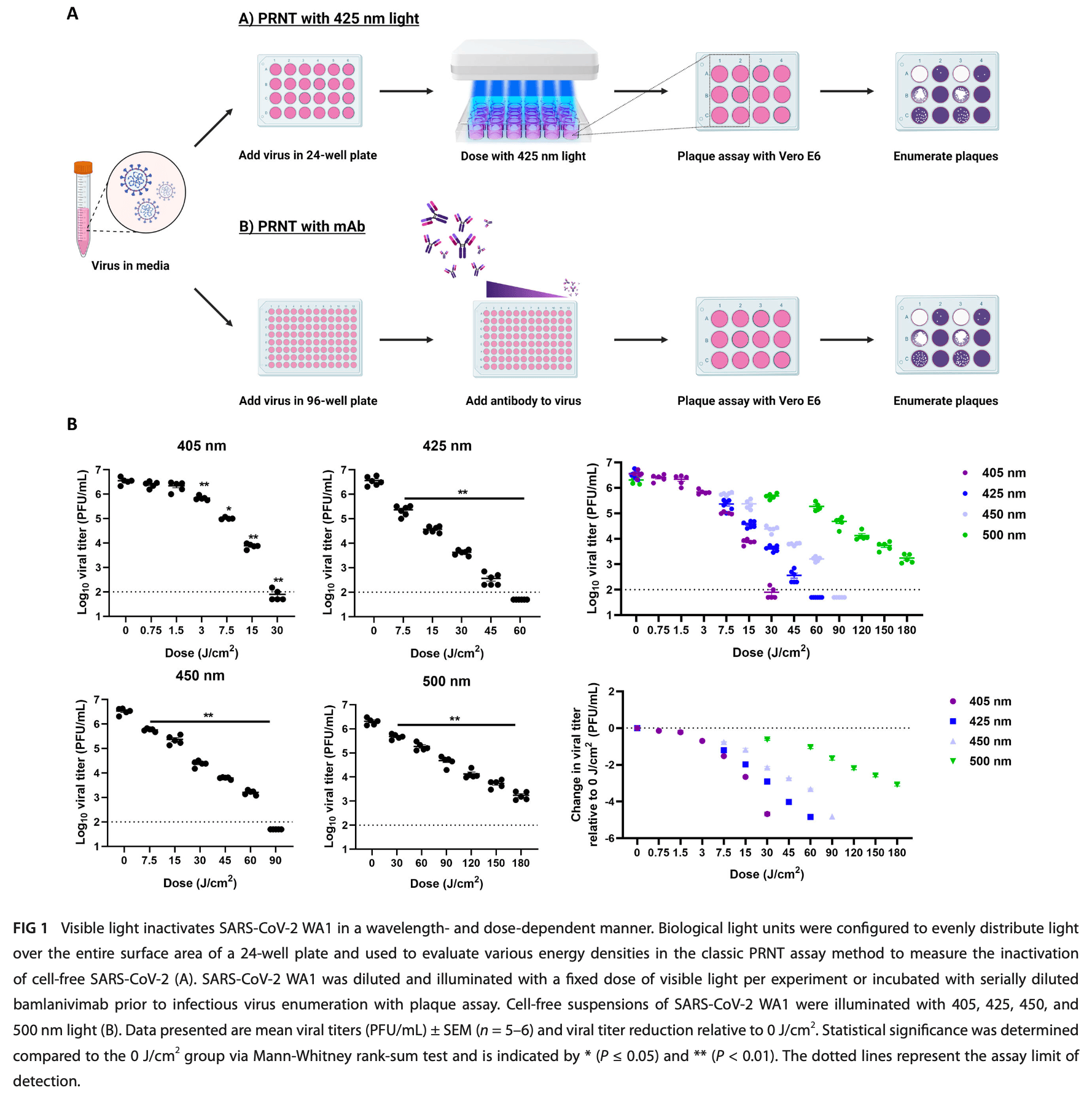
The pan-variant potential of light: 425 nm light inactivates SARS-CoV-2 variants of concern and non-cytotoxic doses reduce viral titers in human airway epithelial cells
et al., mSphere, doi:10.1128/msphere.00230-25, Jun 2025
In vitro study showing that 425 nm visible light inactivates all five SARS-CoV-2 variants of concern (Alpha, Beta, Delta, Gamma, and Omicron) in cell-free suspensions and reduces viral titers in human airway epithelial cells. 60 J/cm² of 425 nm light reduced SARS-CoV-2 titers by >4 log₁₀ across all variants. In well-differentiated air-liquid interface human airway epithelial (ALI HAE) cells, non-cytotoxic doses of 32 J/cm² twice daily reduced infectious virus titers for Beta, Delta, and Omicron variants by 2-5 log₁₀. Riboflavin augmented the antiviral effect, suggesting both photobiomodulation and photodynamic therapy mechanisms.
Stasko et al., 25 Jun 2025, peer-reviewed, 23 authors.
Contact: jkocher@knowbiollc.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
The pan-variant potential of light: 425 nm light inactivates SARS-CoV-2 variants of concern and non-cytotoxic doses reduce viral titers in human airway epithelial cells
mSphere, doi:10.1128/msphere.00230-25
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) prolonged the coronavirus disease 2019 (COVID-19) pandemic. The continued development of novel pan-variant therapeutics to treat currently circulating and future VOCs is critically important. Photomedicine may offer broadly applicable, pan-variant treatments. In this study, we show that visible light centered around 425 nm inactivates each of the five SARS-CoV-2 VOC lineages that have been identified by the World Health Organization (Alpha, Beta, Delta, Gamma, and Omicron) in cell-free suspensions in a dose-dependent manner, including bamlanivimab-resistant variants. Specifically, 60 J/cm 2 of 425 nm light reduced SARS-CoV-2 titers by >4 log 10 relative to unilluminated controls. We observed that 425 nm light inactivates SARS-CoV-2 through restricted entry to host cells. In addition, a non-cytotoxic dosing regimen of 32 J/cm 2 of 425 nm light reduced infectious virus titers in well-differentiated air-liquid interface (ALI) human airway epithelial (HAE) cells infected with the Beta, Delta, and Omicron variants that incorporate mutations associated with immune evasion and/or increased transmissibility. Infectious SARS-CoV-2 titers were reduced when dosing began during the early stages of infection or in more established infections. Finally, we translated these findings to the RD-X19, a novel medical device that emits 425 nm light; our results showed that the RD-X19 restricted spike binding to ACE-2 and reduced SARS-CoV-2 titers in cell-free suspensions (by >2 log 10 ) and in the ALI HAE model (by >1 log 10 ). These findings indicate that photomedicine utilizing 425 nm visible light may serve as a novel, pan-variant treatment modality for COVID-19.
IMPORTANCE The continued spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the emergence of variants that can evade public health measures, including vaccines and therapeutics. Thus, the continued development of broadly applicable measures to supplement current public health measures and standards of care remains critical. Photomedicine is one such approach. In this study, we show that non-ultraviolet visible light can inactivate each SARS-CoV-2 variant of concern (VOC) by preventing entry to host cells. Furthermore, visible light reduced the amount of virus produced in an infection model of the human airway at multiple stages of infection, demonstrating the antiviral capability of visible light. This study provides preclinical support for the development of visible light to serve as a SARS-CoV-2 countermeasure and warrants further investigation.
AUTHOR AFFILIATIONS 1 EmitBio Inc, Morrisville, North Carolina, USA 2 The Marsico Lung Institute, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
ADDITIONAL FILES The following material is available online.
Supplemental Material
References
Abdelnabi, Foo, Jochmans, Vangeel, Jonghe et al., The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern, Nat Commun, doi:10.1038/s41467-022-28354-0
Banaś, Zgłobicki, Kowalska, Bażant, Dziga et al., All you need is light. Photorepair of UV-induced pyrimidine dimers, Genes (Basel), doi:10.1128/AAC.00989-10
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1001/jama.2020.10245
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1001/jama.2020.10245
Cao, Wang, Jian, Song, Yisimayi et al., Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Immunology, doi:10.1101/2021.12.07.470392
Cele, Gazy, Jackson, Hwa, Tegally et al., Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma, Nature, doi:10.1038/s41586-021-03471-w
Chaudhari, Joshi, Kumar, Patel, Lokhande et al., Evaluation of immune evasion in SARS-CoV-2 Delta and Omicron variants, Comput Struct Biotechnol J, doi:10.1038/s41586-021-03471-w
Chaudhari, Kumar, Joshi, Patel, Joshi, E156G and Arg158, Phe-157/del mutation in NTD of spike protein in B.1.617.2 lineage of SARS-CoV-2 leads to immune evasion through antibody escape, bioRxiv, doi:10.1038/s41586-021-04245-0
Chen, Nirula, Heller, Gottlieb, Boscia et al., SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Cheng, Chao, Li, Wang, Kao et al., D614G substitu tion of SARS-CoV-2 spike protein increases syncytium formation and virus titer via enhanced furin-mediated spike cleavage, mBio, doi:10.1038/s41586-021-03471-w
Daniloski, Jordan, Ilmain, Guo, Bhabha et al., The spike D614G mutation increases SARS-COV-2 infection of multiple human cell types, Elife, doi:10.1038/s41586-021-03471-w
Do, Donckers, Vangeel, Chatterjee, Gallay et al., A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents, Antiviral Res, doi:10.1016/j.antiviral.2021.105122
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med, doi:10.1001/jama.2020.10245
Duguay, Herod, Pringle, Monro, Hetu et al., Photodynamic inactivation of human coronaviruses, Viruses, doi:10.1128/AAC.00989-10
Fulcher, Randell, Human nasal and tracheo-bronchial respiratory epithelial cell culture, Methods Mol Biol, doi:10.1007/978-1-62703-125-7_8
Gisaid, GISAID -NextStrain, doi:10.1001/jama.2020.10245
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10245
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Guo, Pan, Mao, Zhang, Wang et al., Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses, Antimicrob Agents Chemother, doi:10.1128/AAC.00989-10
Gómez-López, Jubinville, Rodríguez-López, Trudel-Ferland, Bouchard et al., Inactivation of foodborne viruses by UV light: a review, Foods, doi:10.1128/AAC.00989-10
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1001/jama.2020.10245
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol, doi:10.1038/s41586-021-03471-w
Heinen, Klöhn, Steinmann, Pfaender, In vitro lung models and their application to study SARS-CoV-2 pathogenesis and disease, Viruses, doi:10.3390/v13050792
Hessling, Lau, Vatter, Review of virus inactivation by visible light, Photonics, doi:10.1128/AAC.00989-10
Holoubek, Salát, Kotouček, Kastl, Vancová et al., Antiviral activity of porphyrins and porphyrin-like compounds against tick-borne encephalitis virus: blockage of the viral entry/fusion machinery by photosensitization-mediated destruction of the viral envelope, Antiviral Res, doi:10.1016/j.biopha.2024.116768
Johnson, Zhou, Lokugamage, Vu, Bopp et al., Nucleocapsid mutations in SARS-CoV-2 augment replication and pathogenesis, PLoS Pathog, doi:10.1371/journal.ppat.1010627
Kozlowski, Swann, Current and future issues in the manufactur ing and development of monoclonal antibodies, Adv Drug Deliv Rev, doi:10.1016/j.addr.2006.05.002
Kumar, Chandele, Sharma, Current status of therapeutic monoclonal antibodies against SARS-CoV-2, PLoS Pathog, doi:10.1038/s41586-021-03471-w
Labs, Fact sheet for patients, parents, and caregivers, doi:10.1001/jama.2020.10245
Leanse, Anjos, Mushtaq, Dai, Antimicrobial blue light: a "magic bullet" for the 21st century and beyond?, Adv Drug Deliv Rev, doi:10.1016/j.addr.2021.114057
Leanse, Marasini, Anjos, Dai, Antimicrobial resistance: is there a 'light' at the end of the tunnel?, Antibiotics, doi:10.3390/antibiotics12091437
Liu, Liu, Johnson, Xia, Ku et al., Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant, Cell Rep, doi:10.1016/j.celrep.2022.110829
Liu, Liu, Plante, Plante, Xie et al., The N501Y spike substitution enhances SARS-CoV-2 infection and transmission, Nature, doi:10.1038/s41586-021-04245-0
Liu, Vanblargan, Bloyet, Rothlauf, Chen et al., Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization, Cell Host Microbe, doi:10.1038/s41586-021-03471-w
Maitra, Cunha, Elenbaas, Bonkovsky, Shavit et al., Porphyrin-induced protein oxidation and aggregation as a mechanism of porphyria-associated cell injury, Cell Mol Gastroenterol Hepatol, doi:10.1016/j.jcmgh.2019.06.006
Marovich, Mascola, Cohen, Monoclonal antibodies for prevention and treatment of COVID-19, JAMA, doi:10.1001/jama.2020.10245
Mccallum, Marco, Lempp, Tortorici, Pinto et al., N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2, Cell, doi:10.1038/s41586-021-03471-w
Mendonça, Cadima-Couto, Buga, Arnaut, Schaberle et al., Repurposing anti-cancer porphyrin derivative drugs to target SARS-CoV-2 envelope, Biomed Pharmacother, doi:10.1016/j.biopha.2024.116768
Meng, Abdullahia, Ferreira, Goonawardanen, Saitoa et al., Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity The CITIID-NIHR BioResource COVID-19 Collaboration*, The Genotype to Phenotype Japan (G2P-Japan) Consortium*, Nature, doi:10.1038/s41586-021-03471-w
Motozono, Toyoda, Zahradnik, Saito, Nasser et al., SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity, Cell Host Microbe, doi:10.1038/s41586-021-03471-w
Mukae, Yotsuyanagi, Ohmagari, Doi, Sakaguchi et al., Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis, doi:10.1093/cid/ciac933
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, doi:10.1128/AAC.00989-10
Plante, Liu, Liu, Xia, Johnson et al., Spike mutation D614G alters SARS-CoV-2 fitness, Nature, doi:10.1038/s41586-021-03471-w
Plastiras, Bouquet, Raczkiewicz, Belouzard, De Fourchambault et al., Virucidal activity of porphyrin-based metal-organic frameworks against highly pathogenic coronaviruses and hepatitis C virus, Mater Today Bio, doi:10.1128/AAC.00989-10
Pruijssers, George, Schäfer, Leist, Gralinksi et al., Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice, Cell Rep, doi:10.1038/s41467-022-28354-0
Rathnasinghe, Jangra, Miorin, Schotsaert, Yahnke et al., The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus, Sci Rep, doi:10.1038/s41598-021-97797-0
Ritchie, Mathieu, Rodes-Guirao, Appel, Giattino et al., Coronavirus pandemic (COVID-19), doi:10.1001/jama.2020.10245
Sadraeian, Zhang, Aavani, Biazar, Viral inactivation by light, eLight, doi:10.1128/AAC.00989-10
Santis, Luca, Näslund, Ehmann, Angelis et al., Rapid inactivation of SARS-CoV-2 with LED irradiation of visible spectrum wavelengths, J Photochem Photobiol, doi:10.1002/jbio.202000496
Sheahan, Sims, Zhou, Graham, Pruijssers et al., An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Sci Transl Med, doi:10.1038/s41467-022-28354-0
Stasko, Cockrell, Kocher, Henson, Emerson et al., A randomized, controlled, feasibility study of RD-X19 in subjects, Research Article mSphere
Stasko, Kocher, Annas, Henson, Seitz et al., Visible blue light inhibits infection and replication of SARS-CoV-2 at doses that are well-tolerated by human respiratory tissue, Sci Rep, doi:10.1038/s41598-021-99917-2
Stockslager, Kocher, Arwood, Stasko, Mcdonald et al., Efficacy and hazards of 425 nm oral cavity light dosing to inactivate SARS-CoV-2, J Dent, doi:10.1016/j.jdent.2022.104203
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant, N Engl J Med, doi:10.1038/s41586-021-03471-w
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2, N Engl J Med, doi:10.1101/2021.12.07.470392
Terrosi, Anichini, Docquier, Savellini, Gandolfo et al., Efficient inactivation of SARS-CoV-2 and other RNA or DNA viruses with blue LED light, Pathogens, doi:10.1038/s41586-021-03471-w
Tomb, Maclean, Coia, Graham, Mcdonald et al., New proof-of-concept in viral inactivation: virucidal efficacy of 405 nm light against feline calicivirus as a model for norovirus decontamination, Food Environ Virol, doi:10.1038/s41586-021-03471-w
Touret, Driouich, Cochin, Petit, Gilles et al., Preclinical evaluation of Imatinib does not support its use as an antiviral drug against SARS-CoV-2, Antiviral Res, doi:10.1016/j.antiviral.2021.105137
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: implications for SARS-CoV-2, Nat Rev Microbiol, doi:10.1038/s41579-020-00468-6
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med, doi:10.1038/s41591-021-01678-y
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Widera, Wilhelm, Hoehl, Pallas, Kohmer et al., Limited neutralization of authentic severe acute respiratory syndrome coronavirus 2 variants carrying E484K in vitro, J Infect Dis, doi:10.1038/s41586-021-03471-w
Wu, Xing, Meng, Fu, Xue et al., Nucleocapsid mutations R203K/ G204R increase the infectivity, fitness, and virulence of SARS-CoV-2, Cell Host Microbe, doi:10.1016/j.chom.2021.11.005
Zein, Selting, Hamblin, Review of light parameters and photobiomodulation efficacy: dive into complexity, J Biomed Opt, doi:10.1128/AAC.00989-10
Zhou, Hill, Sarkar, Tse, Woodburn et al., β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells, J Infect Dis, doi:10.1093/infdis/jiab247
Zupin, Clemente, Fontana, Crovella, Effect of near-infrared and blue laser light on vero E6 cells SARS-CoV-2 infection model, J Biophotonics, doi:10.1002/jbio.202200203
Zupin, Gratton, Fontana, Clemente, Pascolo et al., Blue photobiomodulation LED therapy impacts SARS-CoV-2 by limiting its replication in Vero cells, J Biophotonics, doi:10.1002/jbio.202000496
Zupin, Gratton, Milani, Clemente, Fontana et al., Direct inactivation of SARS-CoV-2 by low level blue photobiomo dulation LED at 470, 454 and 450 nm, J Biophotonics, doi:10.1002/jbio.202100375
DOI record:
{
"DOI": "10.1128/msphere.00230-25",
"ISSN": [
"2379-5042"
],
"URL": "http://dx.doi.org/10.1128/msphere.00230-25",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title/>\n <jats:p>\n Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) prolonged the coronavirus disease 2019 (COVID-19) pandemic. The continued development of novel pan-variant therapeutics to treat currently circulating and future VOCs is critically important. Photomedicine may offer broadly applicable, pan-variant treatments. In this study, we show that visible light centered around 425 nm inactivates each of the five SARS-CoV-2 VOC lineages that have been identified by the World Health Organization (Alpha, Beta, Delta, Gamma, and Omicron) in cell-free suspensions in a dose-dependent manner, including bamlanivimab-resistant variants. Specifically, 60 J/cm\n <jats:sup>2</jats:sup>\n of 425 nm light reduced SARS-CoV-2 titers by >4 log\n <jats:sub>10</jats:sub>\n relative to unilluminated controls. We observed that 425 nm light inactivates SARS-CoV-2 through restricted entry to host cells. In addition, a non-cytotoxic dosing regimen of 32 J/cm\n <jats:sup>2</jats:sup>\n of 425 nm light reduced infectious virus titers in well-differentiated air–liquid interface (ALI) human airway epithelial (HAE) cells infected with the Beta, Delta, and Omicron variants that incorporate mutations associated with immune evasion and/or increased transmissibility. Infectious SARS-CoV-2 titers were reduced when dosing began during the early stages of infection or in more established infections. Finally, we translated these findings to the RD-X19, a novel medical device that emits 425 nm light; our results showed that the RD-X19 restricted spike binding to ACE-2 and reduced SARS-CoV-2 titers in cell-free suspensions (by >2 log\n <jats:sub>10</jats:sub>\n ) and in the ALI HAE model (by >1 log\n <jats:sub>10</jats:sub>\n ). These findings indicate that photomedicine utilizing 425 nm visible light may serve as a novel, pan-variant treatment modality for COVID-19.\n </jats:p>\n <jats:sec>\n <jats:title>\n <jats:bold>IMPORTANCE</jats:bold>\n </jats:title>\n <jats:p>The continued spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the emergence of variants that can evade public health measures, including vaccines and therapeutics. Thus, the continued development of broadly applicable measures to supplement current public health measures and standards of care remains critical. Photomedicine is one such approach. In this study, we show that non-ultraviolet visible light can inactivate each SARS-CoV-2 variant of concern (VOC) by preventing entry to host cells. Furthermore, visible light reduced the amount of virus produced in an infection model of the human airway at multiple stages of infection, demonstrating the antiviral capability of visible light. This study provides preclinical support for the development of visible light to serve as a SARS-CoV-2 countermeasure and warrants further investigation.</jats:p>\n </jats:sec>\n </jats:sec>",
"alternative-id": [
"10.1128/msphere.00230-25"
],
"article-number": "e00230-25",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2025-04-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-05-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-05-28"
}
],
"author": [
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Stasko",
"given": "Nathan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Arwood",
"given": "Leslee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Jandick",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Spragion",
"given": "Derry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Roberts",
"given": "Rachel C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Setién",
"given": "Mónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Henson",
"given": "Ibrahim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Annas",
"given": "Abigail",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/0130frc33",
"id-type": "ROR"
}
],
"name": "The Marsico Lung Institute, The University of North Carolina at Chapel Hill",
"place": [
"Chapel Hill, USA"
]
}
],
"family": "Fulcher",
"given": "M. Leslie",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/0130frc33",
"id-type": "ROR"
}
],
"name": "The Marsico Lung Institute, The University of North Carolina at Chapel Hill",
"place": [
"Chapel Hill, USA"
]
}
],
"family": "Brotton",
"given": "Marisa",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Kummer",
"given": "Larry",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Szaba",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Reagan",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Lanzer",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Cookenham",
"given": "Tres",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Casey",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Kothapalli",
"given": "Nagarama",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Hart",
"given": "Tricia",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/04r83e717",
"id-type": "ROR"
}
],
"name": "Trudeau Institute",
"place": [
"Saranac Lake, USA"
]
}
],
"family": "Bradrick",
"given": "Shelton S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Emerson",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"family": "Cockrell",
"given": "Adam S.",
"sequence": "additional"
},
{
"affiliation": [
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/0130frc33",
"id-type": "ROR"
}
],
"name": "The Marsico Lung Institute, The University of North Carolina at Chapel Hill",
"place": [
"Chapel Hill, USA"
]
},
{
"id": [
{
"asserted-by": "publisher",
"id": "https://ror.org/0130frc33",
"id-type": "ROR"
}
],
"name": "Department of Cell Biology and Physiology, The University of North Carolina at Chapel Hill",
"place": [
"Chapel Hill, USA"
]
}
],
"family": "Randell",
"given": "Scott H.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6768-3449",
"affiliation": [
{
"name": "EmitBio Inc",
"place": [
"Morrisville, USA"
]
}
],
"authenticated-orcid": true,
"family": "Kocher",
"given": "Jacob F.",
"sequence": "additional"
}
],
"container-title": "mSphere",
"container-title-short": "mSphere",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2025,
5,
28
]
],
"date-time": "2025-05-28T13:00:16Z",
"timestamp": 1748437216000
},
"deposited": {
"date-parts": [
[
2025,
6,
25
]
],
"date-time": "2025-06-25T13:02:15Z",
"timestamp": 1750856535000
},
"editor": [
{
"affiliation": [],
"family": "Pekosz",
"given": "Andrew",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/100000897",
"award": [
"CFFBOUCHE19R0"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000897",
"id-type": "DOI"
}
],
"name": "Cystic Fibrosis Foundation"
},
{
"DOI": "10.13039/100000062",
"award": [
"P30DK065988"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000062",
"id-type": "DOI"
}
],
"name": "National Institute of Diabetes and Digestive and Kidney Diseases"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
30
]
],
"date-time": "2025-07-30T13:49:56Z",
"timestamp": 1753883396907,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2025,
6,
25
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2025,
6,
25
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
25
]
],
"date-time": "2025-06-25T00:00:00Z",
"timestamp": 1750809600000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
25
]
],
"date-time": "2025-06-25T00:00:00Z",
"timestamp": 1750809600000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/msphere.00230-25",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/msphere.00230-25",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2025,
6,
25
]
]
},
"published-print": {
"date-parts": [
[
2025,
6,
25
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"key": "e_1_3_3_2_2",
"unstructured": "Ritchie H Mathieu E Rodes-Guirao L Appel C Giattino C Ortiz-Ospina E. 2020. Coronavirus pandemic (COVID-19). OurWorldinData.org"
},
{
"key": "e_1_3_3_3_2",
"unstructured": "World Health Organization. 2022. Tracking SARS-CoV-2 variants. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Retrieved 17 Apr 2022."
},
{
"key": "e_1_3_3_4_2",
"unstructured": "GISAID. 2022. GISAID - NextStrain. Available from: https://www.gisaid.org/phylodynamics/global/nextstrain/. Retrieved 24 May 2022."
},
{
"key": "e_1_3_3_5_2",
"unstructured": "Centers for Disease Control and Prevention. 2022. CDC COVID data tracker: variants and genomic surveillance. Available from: https://covid.cdc.gov/covid-data-tracker/#variants-genomic-surveillance. Retrieved 24 May 2022."
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_2"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_9_2"
},
{
"DOI": "10.1056/NEJMoa2102685",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_10_2"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_2"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_12_2"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"DOI": "10.1093/cid/ciac933",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_14_2"
},
{
"DOI": "10.1001/jama.2020.10245",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"DOI": "10.1093/infdis/jiab247",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_16_2"
},
{
"DOI": "10.1016/j.addr.2006.05.002",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_17_2"
},
{
"key": "e_1_3_3_18_2",
"unstructured": "Fact Sheet for Patients And Caregivers Emergency Use Authorization (EUA) Of LAGEVRIO (molnupiravir) capsules. 2022"
},
{
"key": "e_1_3_3_19_2",
"unstructured": "Labs P. 2022. Fact sheet for patients parents and caregivers. Available from: https://www.covid19oralrx-patient.com/files/Fact_Sheet_Patient.pdf. Retrieved 2 May 2022."
},
{
"DOI": "10.1038/s41591-021-01678-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_20_2"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_21_2"
},
{
"DOI": "10.1101/2021.12.07.470392",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_22_2",
"unstructured": "Cao Y Wang J Jian F Xiao T Song W Yisimayi A Huang W Li Q Wang P An R et al.. 2021. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Immunology. doi:10.1101/2021.12.07.470392"
},
{
"DOI": "10.1016/j.jdent.2022.104203",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_23_2"
},
{
"DOI": "10.1038/s41598-021-99917-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_24_2"
},
{
"DOI": "10.1038/s41598-021-97797-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_25_2"
},
{
"DOI": "10.1016/j.jpap.2021.100082",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_26_2"
},
{
"DOI": "10.1002/jbio.202000496",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_27_2"
},
{
"DOI": "10.1111/cts.13249",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_28_2"
},
{
"DOI": "10.1007/978-1-62703-125-7_8",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_29_2"
},
{
"DOI": "10.1056/NEJMc2119407",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_30_2"
},
{
"DOI": "10.1371/journal.ppat.1009885",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_31_2"
},
{
"DOI": "10.1007/s12560-016-9275-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_32_2"
},
{
"DOI": "10.1128/mBio.00587-21",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_33_2"
},
{
"DOI": "10.7554/eLife.65365",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_34_2"
},
{
"DOI": "10.1038/s41586-020-2895-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_35_2"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_36_2"
},
{
"DOI": "10.3390/pathogens10121590",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_37_2"
},
{
"DOI": "10.1016/j.cell.2021.03.028",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_38_2"
},
{
"DOI": "10.1016/j.csbj.2022.08.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_39_2"
},
{
"DOI": "10.1016/j.chom.2021.06.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_40_2"
},
{
"DOI": "10.1093/infdis/jiab355",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_41_2"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_42_2"
},
{
"DOI": "10.1016/j.chom.2021.01.014",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_43_2"
},
{
"DOI": "10.1038/s41586-021-03471-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_44_2"
},
{
"DOI": "10.1101/2021.06.07.447321",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_45_2",
"unstructured": "Chaudhari AM Kumar D Joshi M Patel A Joshi C. 2021. E156G and Arg158 Phe-157/del mutation in NTD of spike protein in B.1.617.2 lineage of SARS-CoV-2 leads to immune evasion through antibody escape. bioRxiv. doi:10.1101/2021.06.07.447321"
},
{
"DOI": "10.1038/s41586-021-04245-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_46_2"
},
{
"DOI": "10.1016/j.celrep.2022.110829",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_47_2"
},
{
"DOI": "10.1016/j.chom.2021.11.005",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_48_2"
},
{
"DOI": "10.1371/journal.ppat.1010627",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_49_2"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_50_2"
},
{
"DOI": "10.3390/foods10123141",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_51_2"
},
{
"DOI": "10.3390/genes11111304",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_52_2"
},
{
"DOI": "10.1186/s43593-022-00029-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_53_2"
},
{
"DOI": "10.3390/photonics9020113",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_54_2"
},
{
"DOI": "10.1117/1.JBO.23.12.120901",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_55_2"
},
{
"DOI": "10.3390/v14010110",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_56_2"
},
{
"DOI": "10.1016/j.mtbio.2024.101165",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_57_2"
},
{
"DOI": "10.1128/AAC.00989-10",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_58_2"
},
{
"DOI": "10.1016/j.antiviral.2023.105767",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_59_2"
},
{
"DOI": "10.1016/j.biopha.2024.116768",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_60_2"
},
{
"DOI": "10.3390/antibiotics12091437",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_61_2"
},
{
"DOI": "10.1016/j.addr.2021.114057",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_62_2"
},
{
"DOI": "10.1038/s41579-020-00468-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_63_2"
},
{
"DOI": "10.1016/j.jcmgh.2019.06.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_64_2"
},
{
"DOI": "10.1002/jbio.202100375",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_65_2"
},
{
"DOI": "10.1002/jbio.202200203",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_66_2"
},
{
"DOI": "10.1016/j.antiviral.2021.105137",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_67_2"
},
{
"DOI": "10.3390/v13050792",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_68_2"
},
{
"DOI": "10.1016/j.antiviral.2021.105122",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_69_2"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_70_2"
},
{
"DOI": "10.1016/j.celrep.2020.107940",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_71_2"
},
{
"DOI": "10.1038/s41467-022-28354-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_72_2"
}
],
"reference-count": 71,
"references-count": 71,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/msphere.00230-25"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The pan-variant potential of light: 425 nm light inactivates SARS-CoV-2 variants of concern and non-cytotoxic doses reduce viral titers in human airway epithelial cells",
"type": "journal-article",
"update-policy": "https://doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "10"
}