A Triple-blind randomized controlled trial on the effects of turmeric versus ginger on inflammatory biomarkers in patients with COVID-19
et al., Scientific Reports, doi:10.1038/s41598-025-16092-4, IRCT20120109008665N14, Aug 2025
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
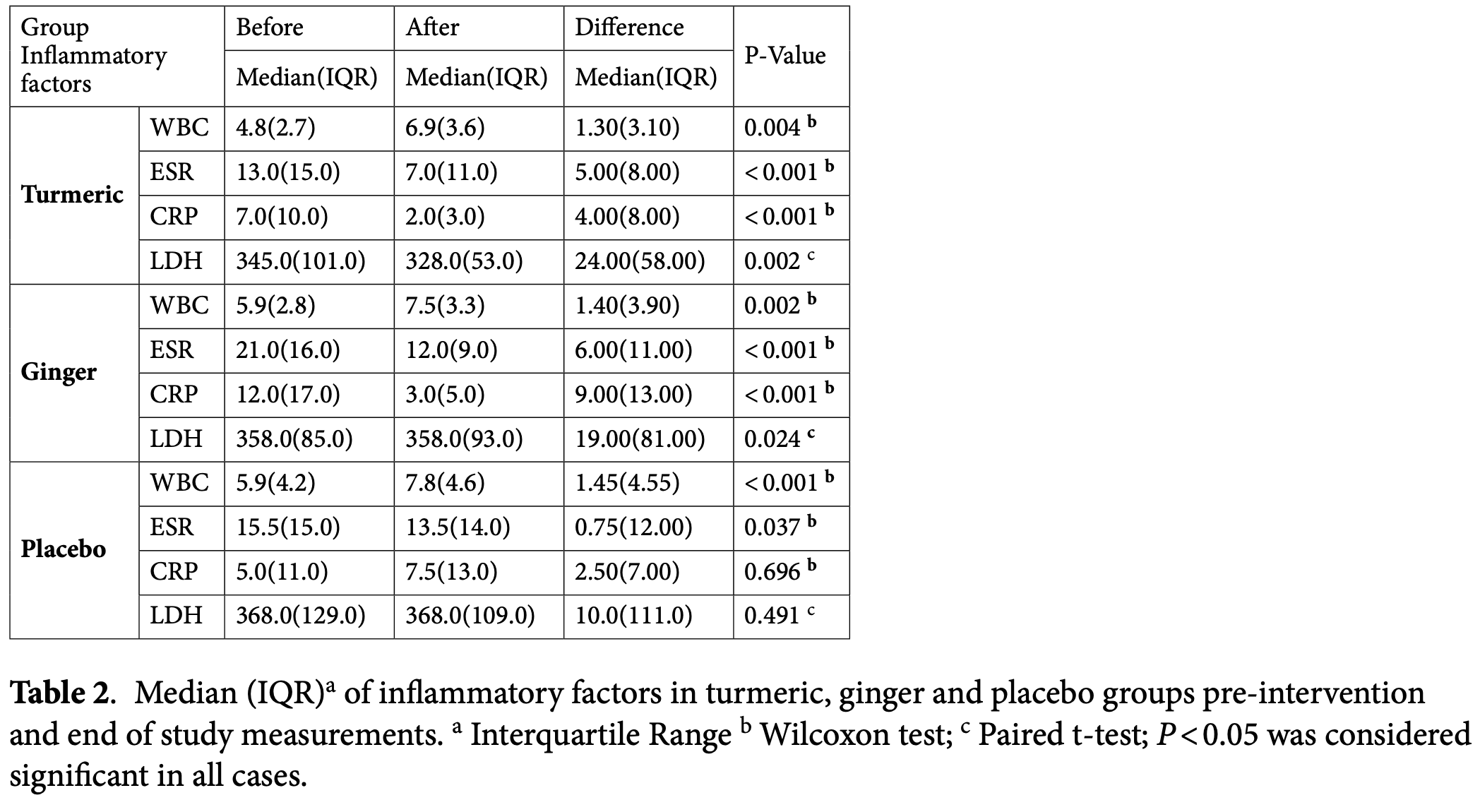
RCT 144 COVID-19 outpatients showing a significant reduction in inflammatory markers (CRP and ESR) with both turmeric and ginger compared with placebo. There was no significant difference between groups for LDH or WBC. Baseline age differed across groups (p=0.023) without adjustment, 16/144 (11%) did not complete testing; clinical outcomes were not analyzed; and there was no followup beyond day 6.
Saleh et al., 21 Aug 2025, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 4 authors, study period 20 November, 2021 - 30 June, 2022, trial IRCT20120109008665N14.
Contact: babamohamadi@semums.ac.ir, babamohammady2007@gmail.com.
A Triple-blind randomized controlled trial on the effects of turmeric versus ginger on inflammatory biomarkers in patients with COVID-19
Scientific Reports, doi:10.1038/s41598-025-16092-4
Inflammation and immune mediators exacerbate COVID-19; turmeric and ginger possess antiviral and anti-inflammatory properties that may mitigate this response. This study compared the effects of turmeric and ginger on inflammatory markers in patients with COVID-19. This triple-blind randomized clinical trial enrolled 144 COVID-19 outpatients at Kowsar Hospital (Semnan, Iran) in 2021. Participants were randomly allocated to group C (turmeric), group G (ginger), or group P (placebo) using blocked randomization and consumed three 500 mg tablets daily for five days (15 tablets total). Inflammatory markers-lactate dehydrogenase (LDH), C-reactive protein (CRP), white blood cell count (WBC), and erythrocyte sedimentation rate (ESR)-were measured at baseline and on day 6. LDH and WBC levels did not differ significantly among groups (p = 0.349 and p = 0.663, respectively). Changes in CRP and ESR varied significantly across groups (p < 0.001), with greater reductions observed in the turmeric and ginger groups compared to the placebo group (p < 0.001), and no difference between the turmeric and ginger groups (CRP: p = 0.263; ESR: p > 0.99). Turmeric and ginger exerted equivalent anti-inflammatory effects; therefore, either agent may serve as a complementary therapy alongside standard treatment to reduce CRP and ESR in COVID-19 outpatients. Trial registration Iranian Registry of Clinical Trials, Trial No IRCT20120109008665N14. Registered 31/08/2021.
Author contributions ZS, MRA, and HB conceived and designed the study. ZS and HB collected, input, and checked the data. RG analyzed the data. ZS and HB draft the manuscript. HB and MRA revised the manuscript, and HB submitted the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval and consent to participate Under the guidance of principles of the World Medical Association Declaration of Helsinki, it was taken into the first consideration to respect participants' rights and to protect their health and rights. The Ethics Committee of Semnan University of Medical Sciences, Semnan, Iran, approved this study (code: IR.SEMUMS. REC.1400.105). Participants were provided with information about data confidentiality, voluntary participation, and their freedom to withdraw from the study. Then, written informed consent for participation was obtained from all of them.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
References
Ahkam, Hermanto, Alamsyah, Aliyyah, Fatchiyah, Virtual prediction of antiviral potential of ginger (Zingiber officinale) bioactive compounds against Spike and MPro of SARS-CoV2 protein. Berk Penelit Hayati, J. Biol. Res, doi:10.23869/50
Ahmad, iochemistry, safety, pharmacological activities, and clinical applications of turmeric: a mechanistic review, Evidence-Based Complementary and Alternative Medicine, doi:10.1155/2020/7656919
Ahmadi, Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial, Food Sci. Nutr, doi:10.1002/fsn3.2226
Allegra, Anticancer activity of Curcumin and its analogues: preclinical and clinical studies, Cancer Invest, doi:10.1080/07357907.2016.1247166
Askari, The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial, Trials, doi:10.1186/s13063-022-06375-w
Ayustaningwarno, Anjani, Ayu, Fogliano, A critical review of ginger's (Zingiber officinale) antioxidant, antiinflammatory, and Immunomodulatory activities, Front. Nutr, doi:10.3389/fnut.2024
Badanta, García, Jiménez, Lucchetti, De Diego-Cordero, The use of complementary and traditional medicine for the treatment of patients with COVID-19: A systematic review, Explore, doi:10.1016/j.explore.2023.02.005
Bagad, Joseph, Bhaskaran, Agarwal, Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa, Advances in Pharmacological and Pharmaceutical Sciences h t t, doi:10.1155/2013/805756
Bagherniya, The use of Curcumin for the treatment of renal disorders: A systematic review of randomized controlled trials, Adv. Exp. Med. Biol, doi:10.1007/978-3-030-56153-6_19
Bhutada, Rathi, Dasar, Immunity boosting diet during COVID 19, Int. J. Pharm. Sci. Res
Boroumand, Samarghandian, Hashemy, Immunomodulatory, anti-inflammatory, and antioxidant effects of Curcumin, J. Herbmed Pharmacol, doi:10.15171/jhp.2018.33
Chen, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, china: a descriptive study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Cui, Chen, Li, Liu, Wang, Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia, J. Thromb. Haemost, doi:10.1111/jth.14830
Dissanayake, Liyanage, Waliwita, Liyanage, A review on medicinal uses of Zingiber officinale (Ginger), Int. J. Health Sci. Res
Dourado, Will Curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials?, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111578
Goel, Kunnumakkara, Aggarwal, Curcumin as curecumin: from kitchen to clinic, Biochem. Pharmacol, doi:10.1016/j.bcp.2007.08.016
Gupta, Jain, Chauhan, Dewan, Inflammatory markers as early predictors of disease severity in COVID-19 patients admitted to intensive care units: A retrospective observational analysis, Indian J. Crit. Care Med, doi:10.5005/jp-journals-10071-24171
Haridas, Compounds of citrus medica and Zingiber officinale for COVID-19 inhibition: in Silico evidence for cues from ayurveda, Futur J. Pharm. Sci, doi:10.1186/s43094-020-00171-6
Hassaniazad, A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytother Res, doi:10.1002/ptr.7294
Hewlings, Kalman, Curcumin A review of its effects on human health, Foods, doi:10.3390/foods6100092
Honarkar Shafie, Effect of Nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial, Phytother Res, doi:10.1002/ptr.7374
Huang, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet h t t, doi:10.1016/S0140-6736(20)30252-X
Hurley, Antidepressant-like effects of Curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF, Behav. Brain Res, doi:10.3390/v12111242
Jafarzadeh, Nemati, Therapeutic potentials of ginger for treatment of multiple sclerosis: A review with emphasis on its immunomodulatory, anti-inflammatory and anti-oxidative properties, J. Neuroimmunol, doi:10.1016/j.jneuroim.2018.09.003
Jalali, The effects of ginger supplementation on markers of inflammatory and oxidative stress: A systematic review and meta-analysis of clinical trials, Phytother Res, doi:10.1002/ptr.6638
Jennings, Parks, Curcumin as an antiviral agent, Viruses, doi:10.3390/v12111242
Jurenka, Anti-inflammatory properties of curcumin, a major constituent of curcuma longa: a review of preclinical and clinical research, Altern. Med. Rev
Khajebishak, Yaghchian, Mohajeri, Payahoo, Ginger, Zingiber officinal Roscoe): A review of its therapeutic uses based on the perspective of modern science and traditional Persian medicine, J. Islamic Iran. Traditional Med
Khosrojerdi, Mashayekhi, Zare Marzouni, Curcumin (extracted from turmeric) and its therapeutic effects, Jorjani Biomed. J
Kumar, Connors, Farber, Human T cell development, localization, and function throughout life, Immunity, doi:10.1016/j.immuni.2018.01.007
Lu, Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet, doi:10.1016/S0140-6736(20)30251-8
Mahluji, Ostadrahimi, Mobasseri, Ebrahimzade Attari, Payahoo, Antiinflammatory effects of Zingiber offi Cinale in type 2 diabetic patients, Adv. Pharm. Bull, doi:10.5681/apb.2013.044
Minassi, Sánchez-Duffhues, Collado, Muñoz, Appendino, Dissecting the pharmacophore of curcumin. Which structural element is critical for which action?, J. Nat. Prod, doi:10.1021/np400148e
Pawar, Oral Curcumin with Piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial, Front. Pharmacol, doi:10.3389/fphar.2021.669362
Pázmándi, Szöllősi, Fekete, The root causes behind the anti-inflammatory actions of ginger compounds in immune cells, Front. Immunol, doi:10.3389/fimmu.2024.1400956
Qin, Efficacy and safety of turmeric and Curcumin in Lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials, Nutr. J, doi:10.1186/s12937-017-0293-y
Roy, Can concomitant use of zinc and Curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19?, Phytother Res, doi:10.1002/ptr.6766
Saber-Moghaddam, Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial, Phytother Res, doi:10.1002/ptr.7004
Sandhiutami, Enhanced wound healing effect of Areca Catechu L. Ointment via antibacterial activity and Anti-Inflammatory process at grade IIA burns in rats, J. Herbmed Pharmacol, doi:10.34172/jhp.2023.42
Shah, Krishnamurthy, Swine flu and its herbal remedies, Int. J. Eng. Sci
Shahzad, Anderson, Najafzadeh, The antiviral, Anti-Inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection, Nutrients, doi:10.3390/nu12092573
Silveira, COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy??, Front. Pharmacol, doi:10.3389/fphar.2020.581840
Singhal, A review of coronavirus Disease-2019 (COVID-19), Indian J. Pediatr, doi:10.1007/s12098-020-03263-6
Sonam, Samatha, Mansi, Shubham, Raziya, Anti-Inflammatory effects of Zingiber officinale: A comprehensive review of its bioactive compounds and therapeutic potential, Medtigo J. Pharmacol, doi:10.63096/medtigo3061113
Srinivasan, Anti-Inflammatory influences of culinary spices and their bioactives, Food Rev. Int, doi:10.1080/87559129.2020.1839761
Sun, Su, Jiao, Wang, Zhang, T cells in health and disease, Signal. Transduct. Target. Ther, doi:10.1038/s41392-023-01471-y
Terpos, Hematological findings and complications of COVID-19, Am. J. Hematol, doi:10.1002/ajh.25829
Thota, Balan, Sivaramakrishnan, Natural products as home-based prophylactic and symptom management agents in the setting of COVID-19, Phytother Res, doi:10.1002/ptr.6794
Verma, Medicinal properties of turmeric (Curcuma longa L.): A review, Int. J. Chem. Stud
Wen, Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J. Med. Chem, doi:10.1021/jm070295s
White, Ginger, an overview, Am. Fam Physician
White, Pasupuleti, Roman, Li, Hernandez, Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials, Pharmacol. Res, doi:10.1016/j.phrs.2019.104280
Wu, Serna, Gan, Fan, Different patterns of leukocyte immune responses to infection of ancestral SARS-CoV-2 and its variants, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2025.1508120
Xin, Clinical retrospective study on the efficacy of Qingfei Paidu Decoction combined with Western medicine for COVID-19 treatment, Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110500
Xu, -2) outside of Wuhan, China: retrospective case series, doi:10.1136/bmj.m792
Zahedipour, Potential effects of Curcumin in the treatment of COVID-19 infection, Phytother Res, doi:10.1002/ptr.6738
Zeng, Association of inflammatory markers with the severity of COVID-19: A meta-analysis, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.05.055
Zhou, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, china: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736
DOI record:
{
"DOI": "10.1038/s41598-025-16092-4",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-16092-4",
"alternative-id": [
"16092"
],
"article-number": "30793",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 August 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "21 August 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Under the guidance of principles of the World Medical Association Declaration of Helsinki, it was taken into the first consideration to respect participants’ rights and to protect their health and rights. The Ethics Committee of Semnan University of Medical Sciences, Semnan, Iran, approved this study (code: IR.SEMUMS.REC.1400.105). Participants were provided with information about data confidentiality, voluntary participation, and their freedom to withdraw from the study. Then, written informed consent for participation was obtained from all of them."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Saleh",
"given": "Zahra",
"sequence": "first"
},
{
"affiliation": [],
"family": "Asgari",
"given": "Mohammad Reza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghorbani",
"given": "Raheb",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babamohamadi",
"given": "Hassan",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
8,
21
]
],
"date-time": "2025-08-21T15:53:33Z",
"timestamp": 1755791613000
},
"deposited": {
"date-parts": [
[
2025,
8,
21
]
],
"date-time": "2025-08-21T15:53:37Z",
"timestamp": 1755791617000
},
"funder": [
{
"DOI": "10.13039/501100018764",
"award": [
"1891"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100018764",
"id-type": "DOI"
}
],
"name": "Semnan University of Medical Sciences and Health Services"
}
],
"indexed": {
"date-parts": [
[
2025,
8,
21
]
],
"date-time": "2025-08-21T16:10:14Z",
"timestamp": 1755792614349,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
8,
21
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
21
]
],
"date-time": "2025-08-21T00:00:00Z",
"timestamp": 1755734400000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
8,
21
]
],
"date-time": "2025-08-21T00:00:00Z",
"timestamp": 1755734400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-16092-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-16092-4",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-16092-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
8,
21
]
]
},
"published-online": {
"date-parts": [
[
2025,
8,
21
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"author": "N Chen",
"doi-asserted-by": "publisher",
"first-page": "507",
"issue": "10223",
"journal-title": "Lancet",
"key": "16092_CR1",
"unstructured": "Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, china: a descriptive study. Lancet 395 (10223), 507–513. https://doi.org/10.1016/S0140-6736(20)30211-7 (2020).",
"volume": "395",
"year": "2020"
},
{
"key": "16092_CR2",
"unstructured": "https://www.worldometers.info/coronavirus/. Accessed Date Oct. 09, 2022."
},
{
"DOI": "10.3389/fphar.2020.581840",
"author": "D Silveira",
"doi-asserted-by": "publisher",
"first-page": "581840",
"journal-title": "Front. Pharmacol.",
"key": "16092_CR3",
"unstructured": "Silveira, D. et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy?? Front. Pharmacol. 11, 581840. https://doi.org/10.3389/fphar.2020.581840 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1007/s12098-020-03263-6",
"author": "T Singhal",
"doi-asserted-by": "publisher",
"first-page": "281",
"issue": "4",
"journal-title": "Indian J. Pediatr.",
"key": "16092_CR4",
"unstructured": "Singhal, T. A review of coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 87 (4), 281–286. https://doi.org/10.1007/s12098-020-03263-6 (2020).",
"volume": "87",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30252-X",
"author": "C Huang",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "16092_CR5",
"unstructured": "Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet https://doi.org/10.1016/S0140-6736(20)30252-X (2020).",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m792",
"author": "XW Xu",
"doi-asserted-by": "publisher",
"journal-title": "bmj",
"key": "16092_CR6",
"unstructured": "Xu, X. W. et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. bmj https://doi.org/10.1136/bmj.m792 (2020).",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "16092_CR7",
"unstructured": "Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, china: a retrospective cohort study. Lancet 395 (10229), 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3 (2020). Erratum in: Lancet. 2020;395(10229):1038. https://doi.org/10.1016/S0140-6736(20)30638-3.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/ajh.25829",
"author": "E Terpos",
"doi-asserted-by": "publisher",
"first-page": "834",
"issue": "7",
"journal-title": "Am. J. Hematol.",
"key": "16092_CR8",
"unstructured": "Terpos, E. et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 95 (7), 834–847. https://doi.org/10.1002/ajh.25829 (2020).",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1111/jth.14830",
"author": "S Cui",
"doi-asserted-by": "publisher",
"first-page": "1421",
"issue": "6",
"journal-title": "J. Thromb. Haemost",
"key": "16092_CR9",
"unstructured": "Cui, S., Chen, S., Li, X., Liu, S. & Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 18 (6), 1421–1424. https://doi.org/10.1111/jth.14830 (2020).",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2021.111578",
"author": "D Dourado",
"doi-asserted-by": "publisher",
"first-page": "111578",
"journal-title": "Biomed. Pharmacother",
"key": "16092_CR10",
"unstructured": "Dourado, D. et al. Will Curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomed. Pharmacother. 139, 111578. https://doi.org/10.1016/j.biopha.2021.111578 (2021).",
"volume": "139",
"year": "2021"
},
{
"DOI": "10.1002/ptr.6794",
"author": "SM Thota",
"doi-asserted-by": "publisher",
"first-page": "3148",
"issue": "12",
"journal-title": "Phytother Res.",
"key": "16092_CR11",
"unstructured": "Thota, S. M., Balan, V. & Sivaramakrishnan, V. Natural products as home-based prophylactic and symptom management agents in the setting of COVID-19. Phytother Res. 34 (12), 3148–3167. https://doi.org/10.1002/ptr.6794 (2020).",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2020.110500",
"author": "S Xin",
"doi-asserted-by": "publisher",
"first-page": "110500",
"journal-title": "Biomed. Pharmacother",
"key": "16092_CR12",
"unstructured": "Xin, S. et al. Clinical retrospective study on the efficacy of Qingfei Paidu Decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 129, 110500. https://doi.org/10.1016/j.biopha.2020.110500 (2020).",
"volume": "129",
"year": "2020"
},
{
"author": "Y Khajebishak",
"first-page": "239",
"issue": "3",
"journal-title": "J. Islamic Iran. Traditional Med.",
"key": "16092_CR13",
"unstructured": "Khajebishak, Y., Yaghchian, M., Mohajeri, M. & Payahoo, L. Ginger (Zingiber officinal Roscoe): A review of its therapeutic uses based on the perspective of modern science and traditional Persian medicine. J. Islamic Iran. Traditional Med. 9 (3), 239–250 (2018). (Persian).",
"volume": "9",
"year": "2018"
},
{
"author": "A Khosrojerdi",
"first-page": "1",
"issue": "2",
"journal-title": "Jorjani Biomed. J.",
"key": "16092_CR14",
"unstructured": "Khosrojerdi, A., Mashayekhi, K. & Zare Marzouni, H. Curcumin (extracted from turmeric) and its therapeutic effects. Jorjani Biomed. J. 4 (2), 1–20 (2016). (Persian).",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.1016/j.bcp.2007.08.016",
"author": "A Goel",
"doi-asserted-by": "publisher",
"first-page": "787",
"issue": "4",
"journal-title": "Biochem. Pharmacol.",
"key": "16092_CR15",
"unstructured": "Goel, A., Kunnumakkara, A. B. & Aggarwal, B. B. Curcumin as curecumin: from kitchen to clinic. Biochem. Pharmacol. 75 (4), 787–809. https://doi.org/10.1016/j.bcp.2007.08.016 (2008).",
"volume": "75",
"year": "2008"
},
{
"DOI": "10.1021/np400148e",
"author": "A Minassi",
"doi-asserted-by": "publisher",
"first-page": "1105",
"issue": "6",
"journal-title": "J. Nat. Prod.",
"key": "16092_CR16",
"unstructured": "Minassi, A., Sánchez-Duffhues, G., Collado, J. A., Muñoz, E. & Appendino, G. Dissecting the pharmacophore of curcumin. Which structural element is critical for which action? J. Nat. Prod. 76 (6), 1105–1112. https://doi.org/10.1021/np400148e (2013).",
"volume": "76",
"year": "2013"
},
{
"DOI": "10.15171/jhp.2018.33",
"author": "N Boroumand",
"doi-asserted-by": "publisher",
"first-page": "211",
"issue": "4",
"journal-title": "J. Herbmed Pharmacol.",
"key": "16092_CR17",
"unstructured": "Boroumand, N., Samarghandian, S. & Hashemy, S. I. Immunomodulatory, anti-inflammatory, and antioxidant effects of Curcumin. J. Herbmed Pharmacol. 7 (4), 211–219. https://doi.org/10.15171/jhp.2018.33 (2018).",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1080/07357907.2016.1247166",
"author": "A Allegra",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Cancer Invest.",
"key": "16092_CR18",
"unstructured": "Allegra, A. et al. Anticancer activity of Curcumin and its analogues: preclinical and clinical studies. Cancer Invest. 35 (1), 1–22. https://doi.org/10.1080/07357907.2016.1247166 (2017).",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.1016/j.bbr.2012.10.049",
"author": "LL Hurley",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "Behav. Brain Res.",
"key": "16092_CR19",
"unstructured": "Hurley, L. L. et al. Antidepressant-like effects of Curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav. Brain Res. 239, 27–30. https://doi.org/10.1016/j.bbr.2012.10.049 (2013).",
"volume": "239",
"year": "2013"
},
{
"DOI": "10.3390/v12111242",
"author": "MR Jennings",
"doi-asserted-by": "publisher",
"first-page": "1242",
"issue": "11",
"journal-title": "Viruses",
"key": "16092_CR20",
"unstructured": "Jennings, M. R. & Parks, R. J. Curcumin as an antiviral agent. Viruses 12 (11), 1242. https://doi.org/10.3390/v12111242 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1021/jm070295s",
"author": "CC Wen",
"doi-asserted-by": "publisher",
"first-page": "4087",
"issue": "17",
"journal-title": "J. Med. Chem.",
"key": "16092_CR21",
"unstructured": "Wen, C. C. et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 50 (17), 4087–4095. https://doi.org/10.1021/jm070295s (2007).",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"author": "R Lu",
"doi-asserted-by": "publisher",
"first-page": "565",
"issue": "10224",
"journal-title": "Lancet",
"key": "16092_CR22",
"unstructured": "Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224), 565–574. https://doi.org/10.1016/S0140-6736(20)30251-8 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6738",
"author": "F Zahedipour",
"doi-asserted-by": "publisher",
"first-page": "2911",
"issue": "11",
"journal-title": "Phytother Res.",
"key": "16092_CR23",
"unstructured": "Zahedipour, F. et al. Potential effects of Curcumin in the treatment of COVID-19 infection. Phytother Res. 34 (11), 2911–2920. https://doi.org/10.1002/ptr.6738 (2020).",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6766",
"author": "A Roy",
"doi-asserted-by": "publisher",
"first-page": "2425",
"issue": "10",
"journal-title": "Phytother Res.",
"key": "16092_CR24",
"unstructured": "Roy, A. et al. Can concomitant use of zinc and Curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytother Res. 34 (10), 2425–2428. https://doi.org/10.1002/ptr.6766 (2020).",
"volume": "34",
"year": "2020"
},
{
"key": "16092_CR25",
"unstructured": "White B Ginger: an overview. Am. Fam Physician. 75 (11), 1689–1691 (2007). PMID: 17575660."
},
{
"author": "KGC Dissanayake",
"first-page": "142",
"journal-title": "Int. J. Health Sci. Res.",
"key": "16092_CR26",
"unstructured": "Dissanayake, K. G. C., Liyanage, W. A., Waliwita, C. & Liyanage, R. P. A review on medicinal uses of Zingiber officinale (Ginger). Int. J. Health Sci. Res. 10, 142–148 (2020).",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3390/nu12092573",
"author": "F Shahzad",
"doi-asserted-by": "publisher",
"first-page": "2573",
"issue": "9",
"journal-title": "Nutrients",
"key": "16092_CR27",
"unstructured": "Shahzad, F., Anderson, D. & Najafzadeh, M. The antiviral, Anti-Inflammatory effects of natural medicinal herbs and mushrooms and SARS-CoV-2 infection. Nutrients 12 (9), 2573. https://doi.org/10.3390/nu12092573 (2020).",
"volume": "12",
"year": "2020"
},
{
"author": "A Shah",
"first-page": "68",
"issue": "5",
"journal-title": "Int. J. Eng. Sci.",
"key": "16092_CR28",
"unstructured": "Shah, A. & Krishnamurthy, R. Swine flu and its herbal remedies. Int. J. Eng. Sci. 2 (5), 68–78 (2013).",
"volume": "2",
"year": "2013"
},
{
"DOI": "10.26452/ijrps.v11iSPL1.3089",
"author": "RS Bhutada",
"doi-asserted-by": "crossref",
"first-page": "832",
"journal-title": "Int. J. Pharm. Sci. Res.",
"key": "16092_CR29",
"unstructured": "Bhutada, R. S., Rathi, R. & Dasar, D. Immunity boosting diet during COVID 19. Int. J. Pharm. Sci. Res. 11, 832–838 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.669362",
"author": "KS Pawar",
"doi-asserted-by": "publisher",
"first-page": "669362",
"journal-title": "Front. Pharmacol.",
"key": "16092_CR30",
"unstructured": "Pawar, K. S. et al. Oral Curcumin with Piperine as adjuvant therapy for the treatment of COVID-19: A randomized clinical trial. Front. Pharmacol. 12, 669362. https://doi.org/10.3389/fphar.2021.669362 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7004",
"author": "N Saber-Moghaddam",
"doi-asserted-by": "publisher",
"first-page": "2616",
"issue": "5",
"journal-title": "Phytother Res.",
"key": "16092_CR31",
"unstructured": "Saber-Moghaddam, N. et al. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial. Phytother Res. 35 (5), 2616–2623. https://doi.org/10.1002/ptr.7004 (2021).",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1155/2020/7656919",
"author": "RS Ahmad",
"doi-asserted-by": "publisher",
"journal-title": "Evidence-Based Complementary and Alternative Medicine",
"key": "16092_CR32",
"unstructured": "Ahmad, R. S. et al. iochemistry, safety, pharmacological activities, and clinical applications of turmeric: a mechanistic review. Evidence-Based Complementary and Alternative Medicine https://doi.org/10.1155/2020/7656919 (2020).",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2019.104280",
"author": "CM White",
"doi-asserted-by": "publisher",
"first-page": "104280",
"journal-title": "Pharmacol. Res.",
"key": "16092_CR33",
"unstructured": "White, C. M., Pasupuleti, V., Roman, Y. M., Li, Y. & Hernandez, A. V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 146, 104280. https://doi.org/10.1016/j.phrs.2019.104280 (2019).",
"volume": "146",
"year": "2019"
},
{
"DOI": "10.1016/j.jneuroim.2018.09.003",
"author": "A Jafarzadeh",
"doi-asserted-by": "publisher",
"first-page": "54",
"journal-title": "J. Neuroimmunol.",
"key": "16092_CR34",
"unstructured": "Jafarzadeh, A. & Nemati, M. Therapeutic potentials of ginger for treatment of multiple sclerosis: A review with emphasis on its immunomodulatory, anti-inflammatory and anti-oxidative properties. J. Neuroimmunol. 324, 54–75. https://doi.org/10.1016/j.jneuroim.2018.09.003 (2018).",
"volume": "324",
"year": "2018"
},
{
"DOI": "10.1002/ptr.6638",
"author": "M Jalali",
"doi-asserted-by": "publisher",
"first-page": "1723",
"issue": "8",
"journal-title": "Phytother Res.",
"key": "16092_CR35",
"unstructured": "Jalali, M. et al. The effects of ginger supplementation on markers of inflammatory and oxidative stress: A systematic review and meta-analysis of clinical trials. Phytother Res. 34 (8), 1723–1733. https://doi.org/10.1002/ptr.6638 (2020).",
"volume": "34",
"year": "2020"
},
{
"author": "RK Verma",
"first-page": "1354",
"issue": "4",
"journal-title": "Int. J. Chem. Stud.",
"key": "16092_CR36",
"unstructured": "Verma, R. K. et al. Medicinal properties of turmeric (Curcuma longa L.): A review. Int. J. Chem. Stud. 6 (4), 1354–1357 (2018).",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.1016/j.ijid.2020.05.055",
"author": "F Zeng",
"doi-asserted-by": "publisher",
"first-page": "467",
"journal-title": "Int. J. Infect. Dis.",
"key": "16092_CR37",
"unstructured": "Zeng, F. et al. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 96, 467–474. https://doi.org/10.1016/j.ijid.2020.05.055 (2020).",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.5005/jp-journals-10071-24171",
"author": "D Gupta",
"doi-asserted-by": "publisher",
"first-page": "482",
"issue": "4",
"journal-title": "Indian J. Crit. Care Med.",
"key": "16092_CR38",
"unstructured": "Gupta, D., Jain, A., Chauhan, M. & Dewan, S. Inflammatory markers as early predictors of disease severity in COVID-19 patients admitted to intensive care units: A retrospective observational analysis. Indian J. Crit. Care Med. 26 (4), 482–486. https://doi.org/10.5005/jp-journals-10071-24171 (2022).",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.3390/foods6100092",
"author": "SJ Hewlings",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "10",
"journal-title": "Foods",
"key": "16092_CR39",
"unstructured": "Hewlings, S. J., Kalman, D. S. & Curcumin A review of its effects on human health. Foods 6 (10), 92. https://doi.org/10.3390/foods6100092 (2017).",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.3389/fnut.2024",
"author": "F Ayustaningwarno",
"doi-asserted-by": "publisher",
"first-page": "1364836",
"journal-title": "Front. Nutr.",
"key": "16092_CR40",
"unstructured": "Ayustaningwarno, F., Anjani, G., Ayu, A. M. & Fogliano, V. A critical review of ginger’s (Zingiber officinale) antioxidant, anti-inflammatory, and Immunomodulatory activities. Front. Nutr. 11, 1364836. https://doi.org/10.3389/fnut.2024 (2024).",
"volume": "11",
"year": "2024"
},
{
"DOI": "10.63096/medtigo3061113",
"author": "SBV Sonam",
"doi-asserted-by": "publisher",
"first-page": "e3061113",
"issue": "1",
"journal-title": "Medtigo J. Pharmacol.",
"key": "16092_CR41",
"unstructured": "Sonam, S. B. V., Samatha, A., Mansi, S., Shubham, R. S. & Raziya, B. S. Anti-Inflammatory effects of Zingiber officinale: A comprehensive review of its bioactive compounds and therapeutic potential. Medtigo J. Pharmacol. 1 (1), e3061113. https://doi.org/10.63096/medtigo3061113 (2024).",
"volume": "1",
"year": "2024"
},
{
"author": "AH Ahkam",
"first-page": "52",
"issue": "2",
"journal-title": "Berk Penelit Hayati J. Biol. Res.",
"key": "16092_CR42",
"unstructured": "Ahkam, A. H., Hermanto, F. E., Alamsyah, A., Aliyyah, I. H. & Fatchiyah, F. Virtual prediction of antiviral potential of ginger (Zingiber officinale) bioactive compounds against Spike and MPro of SARS-CoV2 protein. Berk Penelit Hayati J. Biol. Res. 25 (2), 52–57. https://doi.org/10.23869/50 (2020).",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1186/s43094-020-00171-6",
"author": "M Haridas",
"doi-asserted-by": "publisher",
"first-page": "13",
"issue": "1",
"journal-title": "Futur J. Pharm. Sci.",
"key": "16092_CR43",
"unstructured": "Haridas, M. et al. Compounds of citrus medica and Zingiber officinale for COVID-19 inhibition: in Silico evidence for cues from ayurveda. Futur J. Pharm. Sci. 7 (1), 13. https://doi.org/10.1186/s43094-020-00171-6 (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1155/2013/805756",
"author": "AS Bagad",
"doi-asserted-by": "publisher",
"journal-title": "Advances in Pharmacological and Pharmaceutical Sciences",
"key": "16092_CR44",
"unstructured": "Bagad, A. S., Joseph, J. A., Bhaskaran, N. & Agarwal, A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of Curcuma longa. Advances in Pharmacological and Pharmaceutical Sciences https://doi.org/10.1155/2013/805756 (2013).",
"year": "2013"
},
{
"DOI": "10.1080/87559129.2020.1839761",
"author": "K Srinivasan",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "sup 1",
"journal-title": "Food Rev. Int.",
"key": "16092_CR45",
"unstructured": "Srinivasan, K. Anti-Inflammatory influences of culinary spices and their bioactives. Food Rev. Int. 38 (sup 1), 1–17. https://doi.org/10.1080/87559129.2020.1839761 (2020).",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.34172/jhp.2023.42",
"author": "NMD Sandhiutami",
"doi-asserted-by": "publisher",
"first-page": "388",
"issue": "3",
"journal-title": "J. Herbmed Pharmacol.",
"key": "16092_CR46",
"unstructured": "Sandhiutami, N. M. D. et al. Enhanced wound healing effect of Areca Catechu L. Ointment via antibacterial activity and Anti-Inflammatory process at grade IIA burns in rats. J. Herbmed Pharmacol. 12 (3), 388–398. https://doi.org/10.34172/jhp.2023.42 (2023).",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1007/978-3-030-56153-6_19",
"author": "M Bagherniya",
"doi-asserted-by": "publisher",
"first-page": "327",
"journal-title": "Adv. Exp. Med. Biol.",
"key": "16092_CR47",
"unstructured": "Bagherniya, M. et al. The use of Curcumin for the treatment of renal disorders: A systematic review of randomized controlled trials. Adv. Exp. Med. Biol. 1291, 327–343. https://doi.org/10.1007/978-3-030-56153-6_19 (2021).",
"volume": "1291",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7294",
"author": "M Hassaniazad",
"doi-asserted-by": "publisher",
"first-page": "6417",
"issue": "11",
"journal-title": "Phytother Res.",
"key": "16092_CR48",
"unstructured": "Hassaniazad, M. et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res. 35 (11), 6417–6427. https://doi.org/10.1002/ptr.7294 (2021).",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1002/fsn3.2226",
"author": "R Ahmadi",
"doi-asserted-by": "publisher",
"first-page": "4068",
"issue": "8",
"journal-title": "Food Sci. Nutr.",
"key": "16092_CR49",
"unstructured": "Ahmadi, R. et al. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci. Nutr. 9 (8), 4068–4075. https://doi.org/10.1002/fsn3.2226 (2021).",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7374",
"author": "E Honarkar Shafie",
"doi-asserted-by": "publisher",
"first-page": "1013",
"issue": "2",
"journal-title": "Phytother Res.",
"key": "16092_CR50",
"unstructured": "Honarkar Shafie, E. et al. Effect of Nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial. Phytother Res. 36 (2), 1013–1022. https://doi.org/10.1002/ptr.7374 (2022).",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.5681/apb.2013.044",
"author": "S Mahluji",
"doi-asserted-by": "publisher",
"first-page": "273",
"issue": "2",
"journal-title": "Adv. Pharm. Bull.",
"key": "16092_CR51",
"unstructured": "Mahluji, S., Ostadrahimi, A., Mobasseri, M., Ebrahimzade Attari, V. & Payahoo, L. Antiinflammatory effects of Zingiber offi Cinale in type 2 diabetic patients. Adv. Pharm. Bull. 3 (2), 273–276. https://doi.org/10.5681/apb.2013.044 (2013).",
"volume": "3",
"year": "2013"
},
{
"DOI": "10.3389/fcimb.2025.1508120",
"author": "Y Wu",
"doi-asserted-by": "publisher",
"first-page": "1508120",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "16092_CR52",
"unstructured": "Wu, Y., Serna, R., Gan, W. & Fan, Z. Different patterns of leukocyte immune responses to infection of ancestral SARS-CoV-2 and its variants. Front. Cell. Infect. Microbiol. 15, 1508120. https://doi.org/10.3389/fcimb.2025.1508120 (2025).",
"volume": "15",
"year": "2025"
},
{
"DOI": "10.1186/s13063-022-06375-w",
"author": "G Askari",
"doi-asserted-by": "publisher",
"first-page": "472",
"issue": "1",
"journal-title": "Trials",
"key": "16092_CR53",
"unstructured": "Askari, G. et al. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial. Trials 23 (1), 472. https://doi.org/10.1186/s13063-022-06375-w (2022).",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2024.1400956",
"author": "K Pázmándi",
"doi-asserted-by": "publisher",
"first-page": "1400956",
"journal-title": "Front. Immunol.",
"key": "16092_CR54",
"unstructured": "Pázmándi, K., Szöllősi, A. G. & Fekete, T. The root causes behind the anti-inflammatory actions of ginger compounds in immune cells. Front. Immunol. 15, 1400956. https://doi.org/10.3389/fimmu.2024.1400956 (2024).",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.explore.2023.02.005",
"author": "B Badanta",
"doi-asserted-by": "publisher",
"first-page": "646",
"issue": "5",
"journal-title": "Explore",
"key": "16092_CR55",
"unstructured": "Badanta, B., García, M. A., Jiménez, Á. E., Lucchetti, G. & de Diego-Cordero, R. The use of complementary and traditional medicine for the treatment of patients with COVID-19: A systematic review. Explore 19 (5), 646–662. https://doi.org/10.1016/j.explore.2023.02.005 (2023).",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1016/j.immuni.2018.01.007",
"author": "BV Kumar",
"doi-asserted-by": "publisher",
"first-page": "202",
"issue": "2",
"journal-title": "Immunity",
"key": "16092_CR56",
"unstructured": "Kumar, B. V., Connors, T. J. & Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 48 (2), 202–213. https://doi.org/10.1016/j.immuni.2018.01.007 (2018).",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1038/s41392-023-01471-y",
"author": "L Sun",
"doi-asserted-by": "publisher",
"first-page": "235",
"issue": "1",
"journal-title": "Signal. Transduct. Target. Ther.",
"key": "16092_CR57",
"unstructured": "Sun, L., Su, Y., Jiao, A., Wang, X. & Zhang, B. T cells in health and disease. Signal. Transduct. Target. Ther. 8 (1), 235. https://doi.org/10.1038/s41392-023-01471-y (2023).",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1186/s12937-017-0293-y",
"author": "S Qin",
"doi-asserted-by": "publisher",
"first-page": "68",
"issue": "1",
"journal-title": "Nutr. J.",
"key": "16092_CR58",
"unstructured": "Qin, S. et al. Efficacy and safety of turmeric and Curcumin in Lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr. J. 16 (1), 68. https://doi.org/10.1186/s12937-017-0293-y (2017).",
"volume": "16",
"year": "2017"
},
{
"author": "JS Jurenka",
"first-page": "141",
"issue": "2",
"journal-title": "Altern. Med. Rev.",
"key": "16092_CR59",
"unstructured": "Jurenka, J. S. Anti-inflammatory properties of curcumin, a major constituent of curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 14 (2), 141–153 (2009). PMID: 19594223.",
"volume": "14",
"year": "2009"
}
],
"reference-count": 59,
"references-count": 59,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-16092-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Triple-blind randomized controlled trial on the effects of turmeric versus ginger on inflammatory biomarkers in patients with COVID-19",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}
