Real-world effectiveness and safety of oral Azvudine versus Paxlovid for COVID-19 in patients with kidney disease: a multicenter, retrospective, cohort study
et al., BMC Infectious Diseases, doi:10.1186/s12879-025-10643-w, Feb 2025
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 657 hospitalized COVID-19 patients with kidney disease showing no significant difference in all-cause mortality or disease progression between azvudine and paxlovid. Subgroup analysis showed lower disease progression with azvudine in moderate COVID-19 patients.
Study covers azvudine and paxlovid.
Rao et al., 25 Feb 2025, retrospective, China, peer-reviewed, 15 authors, study period 5 December, 2022 - 31 January, 2023.
Contact: johnyuem@zzu.edu.cn, fccrenzg@zzu.edu.cn.
Real-world effectiveness and safety of oral Azvudine versus Paxlovid for COVID-19 in patients with kidney disease: a multicenter, retrospective, cohort study
BMC Infectious Diseases, doi:10.1186/s12879-025-10643-w
Background Patients with kidney disease (KD) are at high risk of contracting COVID-19 and developing severe disease. There is still a lack of guidance regarding the treatment of COVID-19 in patients with KD. The safety and effectiveness of Azvudine in treating COVID-19 patients with KD remain unknown.
Methods This study included 32,864 COVID-19 patients from nine centers in Henan Province, China. After applying the exclusion criteria and 2:1 propensity score matching, 438 and 219 participants in the Azvudine and Paxlovid groups, respectively, were subjected to analysis.
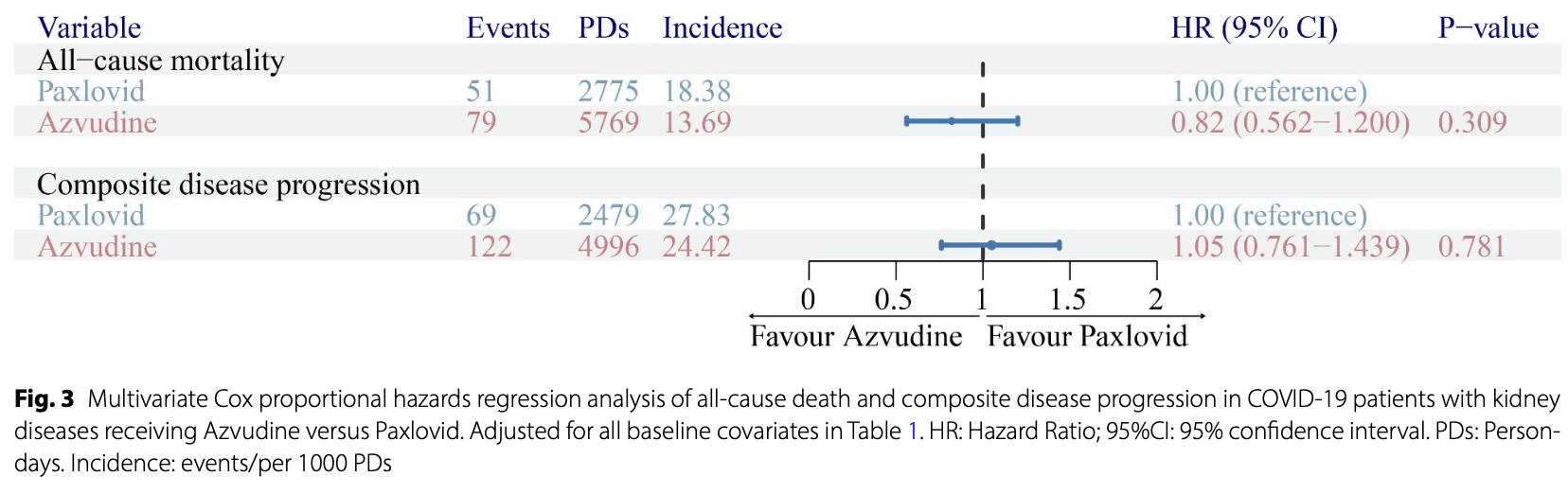
Results Kaplan-Meier analysis revealed no significant differences in all-cause death or composite disease progression between the Azvudine and Paxlovid groups (all p values > 0.05). The same results were obtained in the Cox regression analysis after baseline characteristics adjustment. Three different sensitivity analyses contributed to the robustness of these findings. Subgroup analysis revealed that patients treated with Azvudine had a lower risk of composite disease progression than patients treated with Paxlovid did among patients with moderate disease (p = 0.016, HR: 0.51, 95% CI: 0.27-0.96). Safety data indicated that there was no difference in the incidence of most adverse events. Compared with the Paxlovid group, the Azvudine group had a lower incidence of hypophosphatemia (p = 0.008) and a lower PLT count (p = 0.045). Moreover, during the 15-day follow-up since drug administration, higher concentrations of lymphocytes were detected in the Azvudine group.
Conclusions This study is the first to report that the safety and effectiveness of Azvudine are not inferior to those of Paxlovid in COVID-19 patients with KD. This study provides additional treatment options for COVID-19 patients with KD.
Abbreviations
KD
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 .
Data availability The raw data used and analyzed in this study are included in the Supplementary Tables 2 and Supplementary Table 3 . Further inquiries can be directed to the corresponding author.
Declarations Ethics approval and consent to participate The ethics committee of The First Affiliated Hospital of Zhengzhou University approved this study (2023-KY-0865-001). Considering that this study was a retrospective study, and all patients were anonymous, the ethics committee of the First Affiliated Hospital of Zhengzhou University waived individual informed consent.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Amani, Amani, Azvudine versus Paxlovid in COVID-19: a systematic review and meta-analysis, Rev Med Virol, doi:10.1002/rmv.2551
Amani, Amani, Effectiveness and safety of azvudine in COVID-19: a systematic review and meta-analysis, PLoS ONE, doi:10.1371/journal.pone.0298772
Azzi, Parides, Alani, Loarte-Campos, Bartash et al., COVID-19 infection in kidney transplant recipients at the epicenter of pandemics, Kidney Int, doi:10.1016/j.kint.2020.10.004
Brogan, Ross, The impact of chronic kidney disease on outcomes of patients with COVID-19 admitted to the Intensive Care Unit, Nephron, doi:10.1159/000519530
Cai, Yan, Liu, Li, Ding et al., Paxlovid for hospitalized COVID-19 patients with chronic kidney disease, Antiviral Res, doi:10.1016/j.antiviral.2023.105659
Cai, Yan, Wang, Mou, Efficacy of Paxlovid in patients with acute kidney injury who developed COVID-19, J Infect, doi:10.1016/j.jinf.2022.10.002
Carabelli, Peacock, Thorne, Harvey, Hughes et al., SARS-CoV-2 variant biology: immune escape, transmission and fitness, Nat Rev Microbiol, doi:10.1038/s41579-022-00841-7
China, z h e n g c e / z h e n g c e k u / 2 0 2 2 -0 3 / 1 5 / 5 6 7 9 2 5 7 / fi l e s / 4 9 8 5 4 a 4 9 c 7 0 0 4 e a 9 e 6 2 2
Coca, Singanamala, Parikh, Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis, Kidney Int, doi:10.1038/ki.2011.379
Cowling, The impact of ending 'zero COVID' in China, Nat Med, doi:10.1038/d41591-023-00001-1
Deng, Li, Sun, Zhou, Xiao, Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J Med Virol, doi:10.1002/jmv.28756
Devresse, Sebastien, Greef, Lemaitre, Boland et al., Safety, Efficacy, and relapse of Nirmatrelvir-Ritonavir in kidney transplant recipients infected with SARS-CoV-2, Kidney Int Rep, doi:10.1016/j.ekir.2022.08.026
Dhama, Nainu, Frediansyah, Yatoo, Mohapatra et al., Global emerging Omicron variant of SARS-CoV-2: impacts, challenges and strategies, J Infect Public Health, doi:10.1016/j.jiph.2022.11.024
Fishbane, Hirsch, Nair, Special considerations for Paxlovid Treatment among Transplant recipients with SARS-CoV-2 infection, Am J Kidney Dis, doi:10.1053/j.ajkd.2022.01.001
Francis, Harhay, Ong, Tummalapalli, Ortiz et al., Chronic kidney disease and the global public health agenda: an international consensus, Nat Rev Nephrol, doi:10.1038/s41581-024-00820-6
Gansevoort, Hilbrands, CKD is a key risk factor for COVID-19 mortality, Nat Rev Nephrol, doi:10.1038/s41581-020-00349-4
Goldberg, Lin, Romero-Severson, Ke, Swift and extensive Omicron outbreak in China after sudden exit from 'zero-COVID' policy, Nat Commun, doi:10.1038/s41467-023-39638-4
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for High-Risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Harrington, Cong, Troy, Rawson, 'rear et al., Evaluation of SARS-CoV-2 RNA Rebound after Nirmatrelvir/Ritonavir Treatment in Randomized, Double-Blind, placebo-controlled trials -United States and International sites, 2021-2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7251a2
Hsu, Ordonez, Chertow, Fan, Mcculloch et al., The risk of acute renal failure in patients with chronic kidney disease, Kidney Int, doi:10.1038/ki.2008.107
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Jager, Kovesdy, Langham, Rosenberg, Jha et al., A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases, Kidney Int, doi:10.1016/j.kint.2019.07.012
Lei, Guo, Lu, Qi, Devanathan et al., Activation of PXR causes drug interactions with paxlovid in transgenic mice, Acta Pharm Sin B, doi:10.1016/j.apsb.2023.08.001
Levin, Hemmelgarn, Culleton, Tobe, Mcfarlane et al., Guidelines for the management of chronic kidney disease, CMAJ, doi:10.1503/cmaj.080351
Luo, Zhu, Cai, Zheng, Cheng, Prediction of tacrolimus metabolism and dosage requirements based on CYP3A4 phenotype and CYP3A5(*)3 genotype in Chinese renal transplant recipients, Acta Pharmacol Sin, doi:10.1038/aps.2015.163
Markov, Ghafari, Beer, Lythgoe, Simmonds et al., The evolution of SARS-CoV-2, Nat Rev Microbiol, doi:10.1038/s41579-023-00878-2
Meng, Guo, Feng, Zhao, Zhou et al., Association of CYP3A polymorphisms with the pharmacokinetics of cyclosporine A in early post-renal transplant recipients in China, Acta Pharmacol Sin, doi:10.1038/aps.2012.136
Mutiawati, Kusuma, Fathima, Syahrul, Musadir, A comparison study of headache characteristics and headache-associated quality-of-life of COVID-19 and non-COVID-19 patients, Narra J, doi:10.52225/narra.v2i3.93
Narra, None, doi:10.52225/narra.v2i3.88
Narra, None, doi:10.52225/narra.v2i3.92
Narra, None, doi:10.52225/narra.v4i2.919
Nhcotpsro, COVID-19 diagnosis and treatment plan
Nhcotpsro, Joint Prevention and Control Mechanism of the State Council of China
Organization, Coronavirus disease (COVID-19) Weekly epidemiological update and Weekly operational update
Ren, Luo, Yu, Song, Liang et al., A randomized, Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci (Weinh), doi:10.1002/advs.202001435
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Services Usdohah, Common Terminology Criteria for Adverse Events, CTCAE) Version
Shang, Fu, Geng, Zhang, Zhang et al., Azvudine therapy of common COVID-19 in hemodialysis patients, J Med Virol, doi:10.1002/jmv.29007
Sharun, Tiwari, Yatoo, Natesan, Megawati et al., A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19
Sheng, Li, Li, Wang, Wang et al., Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect, Acta Pharm Sinica B, doi:10.1016/j.apsb.2024.03.032
Varchetta, Mele, Oliviero, Mantovani, Ludovisi et al., Unique immunological profile in patients with COVID-19, Cell Mol Immunol, doi:10.1038/s41423-020-00557-9
Viveiros-Rosa, Mendes, Farfán-Cano, El-Shazly, The race for clinical trials on Omicron-based COVID-19 vaccine candidates: updates from global databases
Wang, Xie, Wang, Fan, Zhang et al., Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and metaanalysis, Virol J, doi:10.1186/s12985-024-02316-y
Yunita, Wahyuni, Sinaga, Yamamoto, Soebandrio et al., Role of ACE2 and TMPRSS2 polymorphisms on COVID-19 outcome and disease severity in adult patients: a prospective cohort study in a tertiary hospital
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymushoming anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00835-6
Zhang, Tan, Ling, Lu, Liu et al., Viral and host factors related to the clinical outcome of COVID-19, Nature, doi:10.1038/s41586-020-2355-0
DOI record:
{
"DOI": "10.1186/s12879-025-10643-w",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-025-10643-w",
"alternative-id": [
"10643"
],
"article-number": "275",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 July 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 February 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "25 February 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The ethics committee of The First Affiliated Hospital of Zhengzhou University approved this study (2023-KY-0865-001). Considering that this study was a retrospective study, and all patients were anonymous, the ethics committee of the First Affiliated Hospital of Zhengzhou University waived individual informed consent."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Rao",
"given": "Benchen",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Daming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Mengzhao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Chunyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Ming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Silin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Donghua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qian",
"given": "Guowu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Shixi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Guotao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Guangming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Zujiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ren",
"given": "Zhigang",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
25
]
],
"date-time": "2025-02-25T19:30:51Z",
"timestamp": 1740511851000
},
"deposited": {
"date-parts": [
[
2025,
2,
25
]
],
"date-time": "2025-02-25T19:31:00Z",
"timestamp": 1740511860000
},
"funder": [
{
"award": [
"2023YFC3043514",
"2022YFC2303100"
],
"name": "National Key Research and Development Program of China"
},
{
"award": [
"ZYCXTD2023002",
"QNCXTD2023002"
],
"name": "Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University"
},
{
"award": [
"HNSWJW-2022013"
],
"name": "Young and middle-aged academic leaders of Henan Provincial Health Commission"
},
{
"award": [
"24HASTIT063"
],
"name": "University Science and Technology Innovation Talent Support Plan of Henan Province"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
26
]
],
"date-time": "2025-02-26T05:32:21Z",
"timestamp": 1740547941720,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
2,
25
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
25
]
],
"date-time": "2025-02-25T00:00:00Z",
"timestamp": 1740441600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
25
]
],
"date-time": "2025-02-25T00:00:00Z",
"timestamp": 1740441600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-10643-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-025-10643-w/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-025-10643-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
2,
25
]
]
},
"published-online": {
"date-parts": [
[
2025,
2,
25
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/ki.2008.107",
"author": "CY Hsu",
"doi-asserted-by": "publisher",
"first-page": "101",
"issue": "1",
"journal-title": "Kidney Int",
"key": "10643_CR1",
"unstructured": "Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–7. https://doi.org/10.1038/ki.2008.107.",
"volume": "74",
"year": "2008"
},
{
"DOI": "10.1016/j.kint.2019.07.012",
"author": "KJ Jager",
"doi-asserted-by": "publisher",
"first-page": "1048",
"issue": "5",
"journal-title": "Kidney Int",
"key": "10643_CR2",
"unstructured": "Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96(5):1048–50. https://doi.org/10.1016/j.kint.2019.07.012.",
"volume": "96",
"year": "2019"
},
{
"DOI": "10.1038/ki.2011.379",
"author": "SG Coca",
"doi-asserted-by": "publisher",
"first-page": "442",
"issue": "5",
"journal-title": "Kidney Int",
"key": "10643_CR3",
"unstructured": "Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8. https://doi.org/10.1038/ki.2011.379.",
"volume": "81",
"year": "2012"
},
{
"DOI": "10.1038/s41581-024-00820-6",
"author": "A Francis",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Nephrol",
"key": "10643_CR4",
"unstructured": "Francis A, Harhay MN, Ong ACM, Tummalapalli SL, Ortiz A, Fogo AB, et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol. 2024. https://doi.org/10.1038/s41581-024-00820-6.",
"year": "2024"
},
{
"DOI": "10.1503/cmaj.080351",
"author": "A Levin",
"doi-asserted-by": "publisher",
"first-page": "1154",
"issue": "11",
"journal-title": "CMAJ",
"key": "10643_CR5",
"unstructured": "Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179(11):1154–62. https://doi.org/10.1503/cmaj.080351.",
"volume": "179",
"year": "2008"
},
{
"key": "10643_CR6",
"unstructured": "Organization WH. Coronavirus disease (COVID-19) Weekly epidemiological update and Weekly operational update, 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. 2020."
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "10643_CR7",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1159/000519530",
"author": "M Brogan",
"doi-asserted-by": "publisher",
"first-page": "67",
"issue": "1",
"journal-title": "Nephron",
"key": "10643_CR8",
"unstructured": "Brogan M, Ross MJ. The impact of chronic kidney disease on outcomes of patients with COVID-19 admitted to the Intensive Care Unit. Nephron. 2022;146(1):67–71. https://doi.org/10.1159/000519530.",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.1038/s41581-020-00349-4",
"author": "RT Gansevoort",
"doi-asserted-by": "publisher",
"first-page": "705",
"issue": "12",
"journal-title": "Nat Rev Nephrol",
"key": "10643_CR9",
"unstructured": "Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16(12):705–6. https://doi.org/10.1038/s41581-020-00349-4.",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.52225/narra.v2i3.92",
"author": "K Sharun",
"doi-asserted-by": "publisher",
"first-page": "e92",
"issue": "3",
"journal-title": "Narra J",
"key": "10643_CR10",
"unstructured": "Sharun K, Tiwari R, Yatoo MI, Natesan S, Megawati D, Singh KP, et al. A comprehensive review on pharmacologic agents, immunotherapies and supportive therapeutics for COVID-19. Narra J. 2022;2(3):e92. https://doi.org/10.52225/narra.v2i3.92.",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1016/j.jiph.2022.11.024",
"doi-asserted-by": "publisher",
"key": "10643_CR11",
"unstructured": "Dhama K, Nainu F, Frediansyah A, Yatoo MI, Mohapatra RK, Chakraborty S, et al. Global emerging Omicron variant of SARS-CoV-2: impacts, challenges and strategies. J Infect Public Health. 2023;16(1). https://doi.org/10.1016/j.jiph.2022.11.024."
},
{
"DOI": "10.52225/narra.v4i2.919",
"author": "R Yunita",
"doi-asserted-by": "publisher",
"first-page": "e919",
"issue": "2",
"journal-title": "Narra J",
"key": "10643_CR12",
"unstructured": "Yunita R, Wahyuni AS, Sinaga BY, Yamamoto Z, Soebandrio A, Kusumawati RL, et al. Role of ACE2 and TMPRSS2 polymorphisms on COVID-19 outcome and disease severity in adult patients: a prospective cohort study in a tertiary hospital, Indonesia. Narra J. 2024;4(2):e919. https://doi.org/10.52225/narra.v4i2.919.",
"volume": "4",
"year": "2024"
},
{
"key": "10643_CR13",
"unstructured": "China, NHCotPsRo. COVID-19 diagnosis and treatment plan (trial version 9). https://www.govcn/zhengce/zhengceku/2022-03/15/5679257/files/49854a49c7004f4ea9e622f3f2c568d8pdf. 2022."
},
{
"key": "10643_CR14",
"unstructured": "China NHCotPsRo. COVID-19 diagnosis and treatment plan (trial version 10). https://www.govcn/zhengce/zhengceku/2023-01/06/5735343/files/5844ce04246b431dbd322d8ba10afb48pdf. 2023."
},
{
"DOI": "10.15585/mmwr.mm7251a2",
"author": "PR Harrington",
"doi-asserted-by": "publisher",
"first-page": "1365",
"issue": "51",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10643_CR15",
"unstructured": "Harrington PR, Cong J, Troy SB, Rawson JMO, O’Rear JJ, Valappil TI, et al. Evaluation of SARS-CoV-2 RNA Rebound after Nirmatrelvir/Ritonavir Treatment in Randomized, Double-Blind, placebo-controlled trials - United States and International sites, 2021–2022. MMWR Morb Mortal Wkly Rep. 2023;72(51):1365–70. https://doi.org/10.15585/mmwr.mm7251a2.",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10643_CR16",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for High-Risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–408. https://doi.org/10.1056/NEJMoa2118542.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Z Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"issue": "19",
"journal-title": "Adv Sci (Weinh)",
"key": "10643_CR17",
"unstructured": "Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, et al. A randomized, Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci (Weinh). 2020;7(19):e2001435. https://doi.org/10.1002/advs.202001435.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "JL Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10643_CR18",
"unstructured": "Zhang JL, Li YH, Wang LL, Liu HQ, Lu SY, Liu Y, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):414. https://doi.org/10.1038/s41392-021-00835-6.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1002/jmv.28756",
"author": "G Deng",
"doi-asserted-by": "publisher",
"first-page": "e28756",
"issue": "4",
"journal-title": "J Med Virol",
"key": "10643_CR19",
"unstructured": "Deng G, Li D, Sun Y, Jin L, Zhou Q, Xiao C, et al. Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J Med Virol. 2023;95(4):e28756. https://doi.org/10.1002/jmv.28756.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2023.105659",
"author": "H Cai",
"doi-asserted-by": "publisher",
"first-page": "105659",
"journal-title": "Antiviral Res",
"key": "10643_CR20",
"unstructured": "Cai H, Yan J, Liu S, Li P, Ding L, Zhan Y, et al. Paxlovid for hospitalized COVID-19 patients with chronic kidney disease. Antiviral Res. 2023;216:105659. https://doi.org/10.1016/j.antiviral.2023.105659.",
"volume": "216",
"year": "2023"
},
{
"key": "10643_CR21",
"unstructured": "SERVICES USDOHAH. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://www.ctepcancergov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7pdf. 2017."
},
{
"DOI": "10.1016/j.apsb.2024.03.032",
"author": "N Sheng",
"doi-asserted-by": "publisher",
"first-page": "3140",
"issue": "7",
"journal-title": "Acta Pharm Sinica B",
"key": "10643_CR22",
"unstructured": "Sheng N, Li R, Li Y, Wang Z, Wang L, Li Y, et al. Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect. Acta Pharm Sinica B. 2024;14(7):3140–54. https://doi.org/10.1016/j.apsb.2024.03.032.",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1002/jmv.29007",
"author": "S Shang",
"doi-asserted-by": "publisher",
"first-page": "e29007",
"issue": "8",
"journal-title": "J Med Virol",
"key": "10643_CR23",
"unstructured": "Shang S, Fu B, Geng Y, Zhang J, Zhang D, Xiao F, et al. Azvudine therapy of common COVID-19 in hemodialysis patients. J Med Virol. 2023;95(8):e29007. https://doi.org/10.1002/jmv.29007.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.52225/narra.v2i3.93",
"author": "E Mutiawati",
"doi-asserted-by": "publisher",
"first-page": "e93",
"issue": "3",
"journal-title": "Narra J",
"key": "10643_CR24",
"unstructured": "Mutiawati E, Kusuma HI, Fathima R, Syahrul S, Musadir N. A comparison study of headache characteristics and headache-associated quality-of-life of COVID-19 and non-COVID-19 patients. Narra J. 2022;2(3):e93. https://doi.org/10.52225/narra.v2i3.93.",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1016/j.kint.2020.10.004",
"author": "Y Azzi",
"doi-asserted-by": "publisher",
"first-page": "1559",
"issue": "6",
"journal-title": "Kidney Int",
"key": "10643_CR25",
"unstructured": "Azzi Y, Parides M, Alani O, Loarte-Campos P, Bartash R, Forest S, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98(6):1559–67. https://doi.org/10.1016/j.kint.2020.10.004.",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2551",
"author": "B Amani",
"doi-asserted-by": "publisher",
"first-page": "e2551",
"issue": "4",
"journal-title": "Rev Med Virol",
"key": "10643_CR26",
"unstructured": "Amani B, Amani B. Azvudine versus Paxlovid in COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2024;34(4):e2551. https://doi.org/10.1002/rmv.2551.",
"volume": "34",
"year": "2024"
},
{
"DOI": "10.1186/s12985-024-02316-y",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "46",
"issue": "1",
"journal-title": "Virol J",
"key": "10643_CR27",
"unstructured": "Wang Y, Xie H, Wang L, Fan J, Zhang Y, Pan S, et al. Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis. Virol J. 2024;21(1):46. https://doi.org/10.1186/s12985-024-02316-y.",
"volume": "21",
"year": "2024"
},
{
"DOI": "10.1371/journal.pone.0298772",
"author": "B Amani",
"doi-asserted-by": "publisher",
"first-page": "e0298772",
"issue": "6",
"journal-title": "PLoS ONE",
"key": "10643_CR28",
"unstructured": "Amani B, Amani B. Effectiveness and safety of azvudine in COVID-19: a systematic review and meta-analysis. PLoS ONE. 2024;19(6):e0298772. https://doi.org/10.1371/journal.pone.0298772.",
"volume": "19",
"year": "2024"
},
{
"DOI": "10.1016/j.jinf.2022.10.002",
"author": "H Cai",
"doi-asserted-by": "publisher",
"first-page": "702",
"issue": "6",
"journal-title": "J Infect",
"key": "10643_CR29",
"unstructured": "Cai H, Yan J, Wang J, Che X, Mou S. Efficacy of Paxlovid in patients with acute kidney injury who developed COVID-19. J Infect. 2022;85(6):702–69. https://doi.org/10.1016/j.jinf.2022.10.002.",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1016/j.ekir.2022.08.026",
"author": "A Devresse",
"doi-asserted-by": "publisher",
"first-page": "2356",
"issue": "11",
"journal-title": "Kidney Int Rep",
"key": "10643_CR30",
"unstructured": "Devresse A, Sebastien B, De Greef J, Lemaitre F, Boland L, Haufroid V, et al. Safety, Efficacy, and relapse of Nirmatrelvir-Ritonavir in kidney transplant recipients infected with SARS-CoV-2. Kidney Int Rep. 2022;7(11):2356–63. https://doi.org/10.1016/j.ekir.2022.08.026.",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1053/j.ajkd.2022.01.001",
"author": "S Fishbane",
"doi-asserted-by": "publisher",
"first-page": "480",
"issue": "4",
"journal-title": "Am J Kidney Dis",
"key": "10643_CR31",
"unstructured": "Fishbane S, Hirsch JS, Nair V. Special considerations for Paxlovid Treatment among Transplant recipients with SARS-CoV-2 infection. Am J Kidney Dis. 2022;79(4):480–2. https://doi.org/10.1053/j.ajkd.2022.01.001.",
"volume": "79",
"year": "2022"
},
{
"DOI": "10.1016/j.apsb.2023.08.001",
"author": "S Lei",
"doi-asserted-by": "publisher",
"first-page": "4502",
"issue": "11",
"journal-title": "Acta Pharm Sin B",
"key": "10643_CR32",
"unstructured": "Lei S, Guo A, Lu J, Qi Q, Devanathan AS, Zhu J, et al. Activation of PXR causes drug interactions with paxlovid in transgenic mice. Acta Pharm Sin B. 2023;13(11):4502–10. https://doi.org/10.1016/j.apsb.2023.08.001.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1038/aps.2012.136",
"author": "XG Meng",
"doi-asserted-by": "publisher",
"first-page": "1563",
"issue": "12",
"journal-title": "Acta Pharmacol Sin",
"key": "10643_CR33",
"unstructured": "Meng XG, Guo CX, Feng GQ, Zhao YC, Zhou BT, Han JL, et al. Association of CYP3A polymorphisms with the pharmacokinetics of cyclosporine A in early post-renal transplant recipients in China. Acta Pharmacol Sin. 2012;33(12):1563–70. https://doi.org/10.1038/aps.2012.136.",
"volume": "33",
"year": "2012"
},
{
"DOI": "10.1038/aps.2015.163",
"author": "X Luo",
"doi-asserted-by": "publisher",
"first-page": "555",
"issue": "4",
"journal-title": "Acta Pharmacol Sin",
"key": "10643_CR34",
"unstructured": "Luo X, Zhu LJ, Cai NF, Zheng LY, Cheng ZN. Prediction of tacrolimus metabolism and dosage requirements based on CYP3A4 phenotype and CYP3A5(*)3 genotype in Chinese renal transplant recipients. Acta Pharmacol Sin. 2016;37(4):555–60. https://doi.org/10.1038/aps.2015.163.",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "publisher",
"first-page": "846",
"issue": "5",
"journal-title": "Intensive Care Med",
"key": "10643_CR35",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–8. https://doi.org/10.1007/s00134-020-05991-x.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/s41423-020-00557-9",
"author": "S Varchetta",
"doi-asserted-by": "publisher",
"first-page": "604",
"issue": "3",
"journal-title": "Cell Mol Immunol",
"key": "10643_CR36",
"unstructured": "Varchetta S, Mele D, Oliviero B, Mantovani S, Ludovisi S, Cerino A, et al. Unique immunological profile in patients with COVID-19. Cell Mol Immunol. 2021;18(3):604–12. https://doi.org/10.1038/s41423-020-00557-9.",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2355-0",
"author": "X Zhang",
"doi-asserted-by": "publisher",
"first-page": "437",
"issue": "7816",
"journal-title": "Nature",
"key": "10643_CR37",
"unstructured": "Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–40. https://doi.org/10.1038/s41586-020-2355-0.",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1038/s41579-023-00878-2",
"author": "PV Markov",
"doi-asserted-by": "publisher",
"first-page": "361",
"issue": "6",
"journal-title": "Nat Rev Microbiol",
"key": "10643_CR38",
"unstructured": "Markov PV, Ghafari M, Beer M, Lythgoe K, Simmonds P, Stilianakis NI, et al. The evolution of SARS-CoV-2. Nat Rev Microbiol. 2023;21(6):361–79. https://doi.org/10.1038/s41579-023-00878-2.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1038/s41579-022-00841-7",
"author": "AM Carabelli",
"doi-asserted-by": "publisher",
"first-page": "162",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "10643_CR39",
"unstructured": "Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, Consortium C-GU, et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21(3):162–77. https://doi.org/10.1038/s41579-022-00841-7.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.52225/narra.v2i3.88",
"author": "SG Viveiros-Rosa",
"doi-asserted-by": "publisher",
"first-page": "e88",
"issue": "3",
"journal-title": "Narra J",
"key": "10643_CR40",
"unstructured": "Viveiros-Rosa SG, Mendes CD, Farfán-Cano GG, El-Shazly M. The race for clinical trials on Omicron-based COVID-19 vaccine candidates: updates from global databases. Narra J. 2022;2(3):e88. https://doi.org/10.52225/narra.v2i3.88.",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-39638-4",
"author": "EE Goldberg",
"doi-asserted-by": "publisher",
"first-page": "3888",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10643_CR41",
"unstructured": "Goldberg EE, Lin Q, Romero-Severson EO, Ke R. Swift and extensive Omicron outbreak in China after sudden exit from ‘zero-COVID’ policy. Nat Commun. 2023;14(1):3888. https://doi.org/10.1038/s41467-023-39638-4.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/d41591-023-00001-1",
"author": "B Cowling",
"doi-asserted-by": "publisher",
"first-page": "302",
"issue": "2",
"journal-title": "Nat Med",
"key": "10643_CR42",
"unstructured": "Cowling B. The impact of ending ‘zero COVID’ in China. Nat Med. 2023;29(2):302. https://doi.org/10.1038/d41591-023-00001-1.",
"volume": "29",
"year": "2023"
},
{
"key": "10643_CR43",
"unstructured": "China NHCotPsRo. Transcript of the press conference held by the Joint Prevention and Control Mechanism of the State Council of China on November 29. 2022. http://www.nhc.gov.cn/xcs/s3574/202211/6fedb556a9324cd3b5b986446ee7ca34.shtml (2022). Accessed November 29, 2022."
},
{
"key": "10643_CR44",
"unstructured": "China NHCotPsRo. COVID-19 vaccination status. http://www.nhc.gov.cn/xcs/yqjzqk/202211/273b32f9a5ed4f9280f24e9ceefbab1d.shtml (2022). Accessed November 29, 2022."
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-025-10643-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-world effectiveness and safety of oral Azvudine versus Paxlovid for COVID-19 in patients with kidney disease: a multicenter, retrospective, cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}
