The Effect of a Low Dose of Vitamin C in Patients With COVID-19: A Double-Blind Randomized Controlled Trial
et al., Disease and Diagnosis, doi:10.34172/ddj.500, Jul 2023
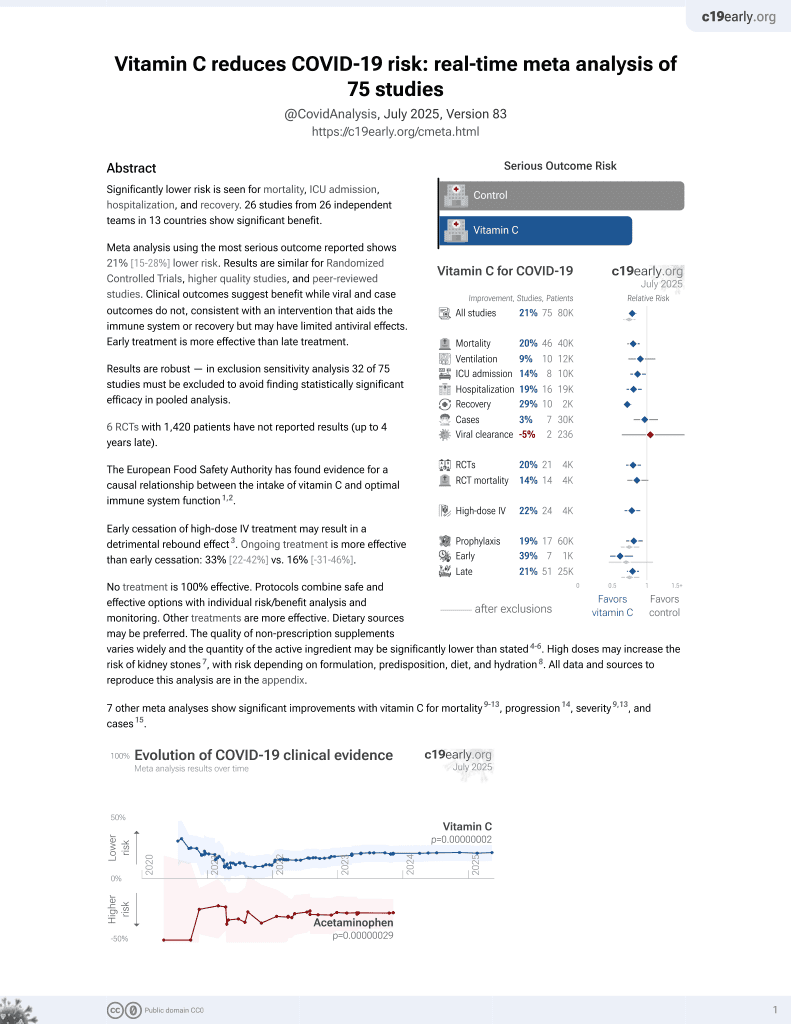
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 401 hospitalized COVID-19 patients showing no significant differences with low-dose oral vitamin C (1000mg daily for 5 days).
Although the 20% lower mortality is not statistically significant, it is consistent with the significant 18% lower mortality [9‑27%] from meta-analysis of the 45 mortality results to date.
This is the 16th of 20 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0016.
This is the 61st of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
|
risk of death, 20.4% lower, RR 0.80, p = 0.64, treatment 8 of 201 (4.0%), control 10 of 200 (5.0%), NNT 98, day 28.
|
|
risk of death, 99.0% higher, RR 1.99, p = 1.00, treatment 2 of 201 (1.0%), control 1 of 200 (0.5%), ICU mortality.
|
|
risk of mechanical ventilation, 199.5% higher, RR 3.00, p = 1.00, treatment 1 of 201 (0.5%), control 0 of 200 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 32.7% higher, RR 1.33, p = 0.79, treatment 8 of 201 (4.0%), control 6 of 200 (3.0%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mousaviasl et al., 22 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 13 authors, study period November 2020 - May 2021, dosage 500mg bid days 1-5.
The Effect of a Low Dose of Vitamin C in Patients With COVID-19: A Double-Blind Randomized Controlled Trial
Disease and Diagnosis, doi:10.34172/ddj.500
Background: Vitamin C is a micronutrient with anti-inflammatory and free radical scavenging properties that can strengthen the body's immune system. In this study, it was attempted to assess the clinical efficiency of oral vitamin C in treating COVID-19. Materials and Methods: This double-blind randomized clinical trial was conducted on 401 patients hospitalized in Taleghani hospital, Abadan, over 18 years of age and with confirmed COVID-19 infection, from November 2020 to May 2021. The patients were randomly assigned to intervention groups (201 people, two tablets per day, each containing 500 mg of vitamin C) and the control group (200 people, placebo, containing starch received for five days). Improvements in clinical symptoms, death from baseline to the 28-day follow-up after the intervention, hospital length of stay, and laboratory values of C-reactive protein (CRP) were some of the considered outcome variables. Results: No significant difference was observed between the two groups in terms of the daily improvement of clinical symptoms and the odds of healing from each symptom increased by about 48-50%. The difference in the length of hospital stay between the two groups was close to significant (P = 0.051). There was no significant difference in mortality between the two groups (P = 0.8). There was no difference between the two groups in the laboratory parameters, except for alkaline phosphatase (P = 0.03). Conclusion: Vitamin C had no significant effect on improving patients' clinical symptoms such as fatigue, fever, cough, and shortness of breath.
Authors' Contribution
Competing Interests The authors declare that they have no conflict of interests regarding the publication of this study.
Disclaimer This article doesn't have prior publication or presentation in a conference/seminar.
Ethical Approval The protocol of this study was approved by Abadan Medical Sciences Ethics Committee with the ethics code IR.ABADANUMS. REC.1398.119. The trial was conducted in accordance with the principles of the Declaration of Helsinki and registered in the Iranian Registry of Clinical Trials (www.irct.ir) on April 4, 2020 (identifier: IRCT20200324046850N5; https://www.irct.ir/).
Informed Consent All participants gave their written informed consent after receiving explanations regarding the study objective and methodology.
References
Bourbour, Dahka, Gholamalizadeh, Akbari, Shadnoush et al., Nutrients in prevention, treatment, and management of viral infections; special focus on coronavirus, Arch Physiol Biochem, doi:10.1080/13813455.2020.1791188
Carr, Rowe, The emerging role of vitamin C in the prevention and treatment of COVID-19, Nutrients, doi:10.3390/nu12113286
Darban, Malek, Memarian, Gohari, Kiani et al., Efficacy of high dose vitamin C, melatonin and zinc in Iranian patients with acute respiratory syndrome due to coronavirus infection: a pilot randomized trial, J Cell Mol Anesth
Fowler Aa 3rd, Truwit, Hite, Morris, Dewilde et al., Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial, JAMA, doi:10.1001/jama.2019.11825
García, Immune response, inflammation, and the clinical spectrum of COVID-19, Front Immunol, doi:10.3389/fimmu.2020.01441
Liu, Zhu, Zhang, Li, Peng, Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial, BMJ Open, doi:10.1136/bmjopen-2020-039519
Lykkesfeldt, Michels, Frei, Vitamin C, Adv Nutr, doi:10.3945/an.113.005157
Lykkesfeldt, Tveden-Nyborg, The pharmacokinetics of vitamin C, Nutrients, doi:10.3390/nu11102412
Majidi, Rabbani, Gholami, Gholamalizadeh, Bourbour et al., The effect of vitamin C on pathological parameters and survival duration of critically ill coronavirus disease 2019 patients: a randomized clinical trial, Front Immunol, doi:10.3389/fimmu.2021.717816
Moghadam Siahkali, Zarezade, Koolaji, Alinaghi, Zendehdel et al., Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial, Eur J Med Res, doi:10.1186/s40001-021-00490-1
Mousavi, Bereswill, Heimesaat, Immunomodulatory and antimicrobial effects of vitamin C, Eur J Microbiol Immunol, doi:10.1556/1886.2019.00016
Munster, Koopmans, Van Doremalen, Van Riel, De, A novel coronavirus emerging in China -key questions for impact assessment, N Engl J Med, doi:10.1056/NEJMp2000929
Rawat, Roy, Maitra, Gulati, Khanna et al., Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.102324
Ried, Binjemain, Sali, Therapies to prevent progression of COVID-19, including hydroxychloroquine, azithromycin, zinc, and vitamin D3 with or without intravenous vitamin C: an international, multicenter, randomized trial, Cureus, doi:10.7759/cureus.19902
Schloss, Lauche, Harnett, Hannan, Brown et al., Efficacy and safety of vitamin C in the management of acute respiratory infection and disease: a rapid review, Adv Integr Med, doi:10.1016/j.aimed.2020.07.008
Tian, Hu, Niu, Liu, Xu et al., Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer, J Thorac Oncol, doi:10.1016/j.jtho.2020.02.010
Vitamin, Fact Sheet for Health Professionals
Xia, Fan, He, Zhu, Zheng, High-dose intravenous vitamin C attenuates hyperinflammation in severe coronavirus disease 2019, Nutrition, doi:10.1016/j.nut.2021.111405
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med, doi:10.1016/s2213-2600(20)30076-x
Zhang, Rao, Li, Zhu, Liu et al., Pilot trial of high-dose vitamin C in critically ill COVID-19 patients, Ann Intensive Care, doi:10.1186/s13613-020-00792-3
DOI record:
{
"DOI": "10.34172/ddj.500",
"ISSN": [
"2717-3232"
],
"URL": "http://dx.doi.org/10.34172/ddj.500",
"abstract": "<jats:p>Background: Vitamin C is a micronutrient with anti-inflammatory and free radical scavenging properties that can strengthen the body’s immune system. In this study, it was attempted to assess the clinical efficiency of oral vitamin C in treating COVID-19. Materials and Methods: This double-blind randomized clinical trial was conducted on 401 patients hospitalized in Taleghani hospital, Abadan, over 18 years of age and with confirmed COVID-19 infection, from November 2020 to May 2021. The patients were randomly assigned to intervention groups (201 people, two tablets per day, each containing 500 mg of vitamin C) and the control group (200 people, placebo, containing starch received for five days). Improvements in clinical symptoms, death from baseline to the 28-day follow-up after the intervention, hospital length of stay, and laboratory values of C-reactive protein (CRP) were some of the considered outcome variables. Results: No significant difference was observed between the two groups in terms of the daily improvement of clinical symptoms and the odds of healing from each symptom increased by about 48-50%. The difference in the length of hospital stay between the two groups was close to significant (P=0.051). There was no significant difference in mortality between the two groups (P=0.8). There was no difference between the two groups in the laboratory parameters, except for alkaline phosphatase (P=0.03). Conclusion: Vitamin C had no significant effect on improving patients’ clinical symptoms such as fatigue, fever, cough, and shortness of breath.</jats:p>",
"assertion": [
{
"label": "Journal Owner",
"name": "journal_owner",
"value": "Hormozgan University of Medical Sciences"
},
{
"label": "Journal Publisher",
"name": "journal_publisher",
"value": "Hormozgan University of Medical Sciences"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2023-03-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2023-06-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2023-07-22"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1854-305X",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Mousaviasl",
"given": "Sajedeh",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0617-6758",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Sayar",
"given": "Sara",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1369-6580",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Radmanesh",
"given": "Esmat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4876-1058",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Pahlavanzade",
"given": "Bagher",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8877-6924",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Esmaeilian",
"given": "Hani",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8109-9297",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Ebrahimzadeh",
"given": "Mona",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6751-3968",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Tabahfar",
"given": "Raha",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8159-7237",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "khalili",
"given": "Maryam",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6570-0532",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Borzoo",
"given": "Tara",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2307-1659",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Jelvay",
"given": "Saeed",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4688-9856",
"affiliation": [
{
"name": "Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran"
}
],
"authenticated-orcid": true,
"family": "Bitaraf",
"given": "Saeid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6035-7464",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Naghashpour",
"given": "Mahshid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4530-1080",
"affiliation": [
{
"name": "Abadan University of Medical Sciences, Abadan, Iran"
}
],
"authenticated-orcid": true,
"family": "Mobarak",
"given": "Sara",
"sequence": "additional"
}
],
"container-title": "Disease and Diagnosis",
"container-title-short": "Dis Diagn",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"ddj.hums.ac.ir"
]
},
"created": {
"date-parts": [
[
2024,
1,
6
]
],
"date-time": "2024-01-06T10:41:06Z",
"timestamp": 1704537666000
},
"deposited": {
"date-parts": [
[
2024,
1,
6
]
],
"date-time": "2024-01-06T10:41:07Z",
"timestamp": 1704537667000
},
"indexed": {
"date-parts": [
[
2024,
1,
7
]
],
"date-time": "2024-01-07T00:16:31Z",
"timestamp": 1704586591791
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
7,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
1,
2
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://ddj.hums.ac.ir/PDF/ddj-13-18.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://ddj.hums.ac.ir/PDF/ddj-13-18.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "20123",
"original-title": [],
"page": "18-25",
"prefix": "10.34172",
"published": {
"date-parts": [
[
2023,
7,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
22
]
]
},
"publisher": "Maad Rayan Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://ddj.hums.ac.ir/Article/ddj-500"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Earth-Surface Processes"
],
"subtitle": [],
"title": "The Effect of a Low Dose of Vitamin C in Patients With COVID-19: A Double-Blind Randomized Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.34172/crossmark_policy",
"volume": "13"
}