Does early combination vs. Monotherapy improve clinical outcomes of clinically extremely vulnerable patients with COVID-19? Results from a retrospective propensity-weighted analysis
et al., European Journal of Medical Research, doi:10.1186/s40001-024-02062-5, Oct 2024
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
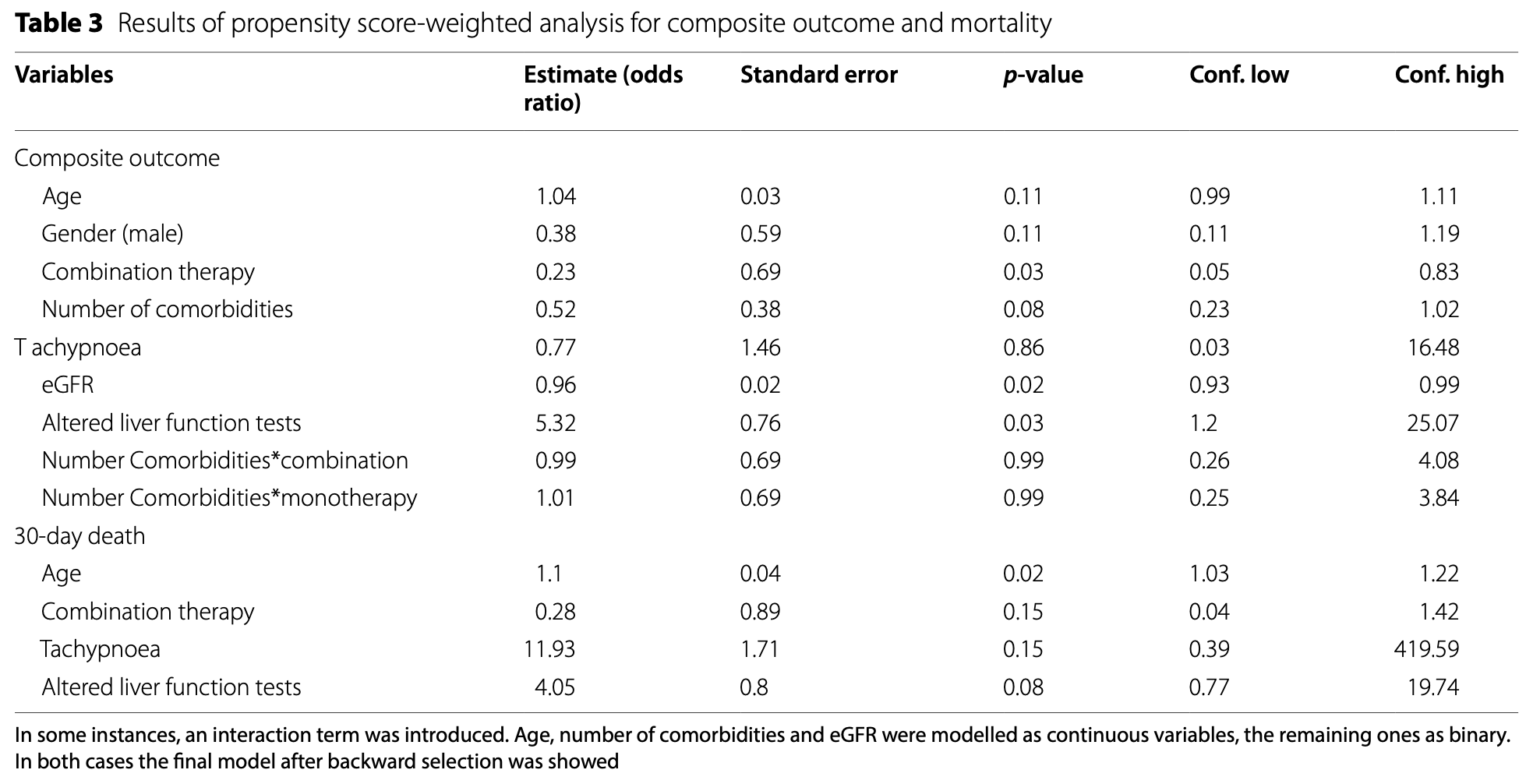
Retrospective 81 severely immunocompromised COVID-19 outpatients in Italy showing improved composite outcome of death, hospitalization, and emergency department encounters with early combination therapy of an antiviral plus sotrovimab compared to antiviral monotherapy.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
|
risk of death, 72.0% lower, OR 0.28, p = 0.15, treatment 39, control 42, propensity score weighting, RR approximated with OR.
|
|
risk of progression, 77.0% lower, OR 0.23, p = 0.03, treatment 39, control 42, propensity score weighting, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Maria et al., 4 Oct 2024, retrospective, Italy, peer-reviewed, 10 authors, study period 1 January, 2022 - 31 December, 2023, average treatment delay 2.0 days.
Contact: m.mazzitelli88@gmail.com.
Does early combination vs. Monotherapy improve clinical outcomes of clinically extremely vulnerable patients with COVID-19? Results from a retrospective propensity-weighted analysis
European Journal of Medical Research, doi:10.1186/s40001-024-02062-5
Background The potential efficacy of early combination therapy, based on an antiviral plus a monoclonal antibody, for COVID-19 in severely immunocompromised patients is matter of debate. Objectives Our aim was to describe the impact on clinical outcomes of COVID-19 treatments in severely immunocompromised individuals, evaluating differences between a combination and a monotherapy.
Methods We included severely immunocompromised outpatients with mild-to-moderate COVID-19 who received an early treatment (either monotherapy with nirmatrelvir/ritonavir or remdesivir or the combination of an antiviral plus sotrovimab). We then assessed differences between the two treatment strategies on three main outcomes (30day mortality, access to emergency department, hospitalization), separately and as a composite by using a propensity score weighted (PSW) approach. Results Eighty one severely immunocompromised patients were included, 39 receiving early combination therapy and 42 receiving monotherapy. No significant difference was observed in the 30-day mortality rate and hospitalization rate between subjects in the two groups, while access to the emergency department following treatment administration was significantly higher in people who received a combination therapy. After applying the PSW, it was observed that combination therapy impacted favourably on the composite outcome, in a statistically significant fashion. In addition, PSW approach for mortality showed that age was the only significant factor influencing the death as stand-alone outcome.
Conclusions Early combination therapy showed a favourable impact on a composite outcome (including mortality, hospitalizations and access to emergency department) in severely immunocompromised hosts who were all vaccinated. However, further studies are needed to support our results.
Supplementary Information The online version contains supplementary material available at https:// doi . org/ 10. 1186/ s40001-024-02062-5.
Supplementary Material 1 Author contributions M.M. conceived the study. A.M. performed statistical analysis. M.M. and A.M wrote the draft of the manuscript. C.C., M.B., L.S., A.F, V.S., N.B. and E.V. curated and collected the data. A.M.C. supervised the project. All authors reviewed, approved the final version of the manuscript, and by having access to all the data had final responsibility for the decision to submit this paper for publication.
Declarations Ethics approval and consent to participate Study protocol was approved by Local Ethic Committee (n. AOP 0002323, January 1rst, 2022). Each patient was requested to sign written informed consent for participation.
Competing interests
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Antinori, Bausch-Jurken, The burden of COVID-19 in the immunocompromised patient: implications for vaccination and needs for the future, J Infect Dis, doi:10.1093/infdis/jiad181
Bai, Beringheli, Vitaletti, Santoro, Mola et al., Clinical outcome and 7-day virological clearance in high-risk patients with mildmoderate COVID-19 treated with molnupiravir, nirmatrelvir/ritonavir, or remdesivir, Infect Dis Ther, doi:10.1007/s40121-024-00994-3
Basoulis, Tsakanikas, Gkoufa, Bitsani, Karamanakos et al., Effectiveness of oral nirmatrelvir/ritonavir vs. intravenous three-day remdesivir in preventing progression to severe COVID-19: a single-center, prospective, comparative, real-life study, Viruses, doi:10.3390/v15071515
Bhimraj, Morgan, Shumaker, Baden, Cheng et al., Infectious diseases society of America Guidelines on the treatment and management of patients with COVID-19, Clin Infect Dis, doi:10.1093/cid/ciac724
Boeckh, Pergam, Limaye, Englund, Corey et al., How immunocompromised hosts were left behind in the quest to control the Covid-19 Pandemic, Clin Infect Dis, doi:10.1093/cid/ciae308
Brookhart, Schneeweiss, Rothman, Glynn, Avorn et al., Variable selection for propensity score models, Am J Epidemiol, doi:10.1093/aje/kwj149
Caso, Fernandez-Ruiz, Lopez-Medrano, Teller, Lizasoain et al., Nirmatrelvir/ritonavir for the treatment of immunocompromised adult patients with early-stage symptomatic COVID-19: a real-life experience, J Med Virol, doi:10.1002/jmv.29082
Castelnuovo, Costanzo, Antinori, Berselli, Blandi et al., Lopinavir/ritonavir and darunavir/cobicistat in hospitalized COVID-19 patients: findings from the multicenter Italian CORIST study, Front Med, doi:10.3389/fmed.2021.639970
Cepeda, Boston, Farrar, Strom, Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders, Am J Epidemiol, doi:10.1093/aje/kwg115
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe, doi:10.1016/S2666-5247(20)30172-5
Chan, Yuan, Chu, Sridhar, Yuen, COVID-19 drug discovery and treatment options, Nat Rev Microbiol, doi:10.1038/s41579-024-01036-y
Chesnaye, Stel, Tripepi, Dekker, Fu et al., An introduction to inverse probability of treatment weighting in observational research, Clin Kidney J, doi:10.1093/ckj/sfab158
Dewolf, Laracy, Perales, Kamboj, Van Den Brink et al., SARS-CoV-2 in immunocompromised individuals, Immunity, doi:10.1016/j.immuni.2022.09.006
Dioverti, Salto-Alejandre, Haidar, Immunocompromised patients with protracted COVID-19: a Review of "Long Persisters, Curr Transplant Rep, doi:10.1007/s40472-022-00385-y
Eastman, Roth, Brimacombe, Simeonov, Shen et al., Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19, ACS Cent Sci, doi:10.1021/acscentsci.0c00489
Evans, Dube, Lu, Yates, Arnetorp et al., Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2023.100747
Feys, Lagrou, Lauwers, Haenen, Jacobs et al., High burden of COVID-19-associated pulmonary aspergillosis in severely immunocompromised patients requiring mechanical ventilation, Clin Infect Dis, doi:10.1093/cid/ciad546
Focosi, Casadevall, Franchini, Sotrovimab: a review of its efficacy against SARS-CoV-2 variants, Viruses, doi:10.3390/v16020217
Focosi, Franchini, Maggi, Shoham, COVID-19 therapeutics, Clin Microbiol Rev, doi:10.1128/cmr.00119-23
Focosi, Maggi, ' Abramo, Nicastri, Sullivan, Antiviral combination therapies for persistent COVID-19 in immunocompromised patients, Int J Infect Dis, doi:10.1016/j.ijid.2023.09.021
Garrido, Propensity scores: a practical method for assessing treatment effects in pain and symptom management research, J Pain Symptom Manage, doi:10.1016/j.jpainsymman.2014.05.014
Gentile, Foggia, Silvitelli, Sardanelli, Cattaneo et al., Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab, Virol J, doi:10.1186/s12985-023-02269-8
Gilbert, Montefiori, Mcdermott, Fong, Benkeser et al., Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial, Science, doi:10.1126/science.abm3425
Godwin, Polsonetti, Caron, Oppelt, Remdesivir for the treatment of COVID-19: a narrative review, Infect Dis Ther, doi:10.1007/s40121-023-00900-3
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of bamlanivimab as monotherapy or in combination with etesevimab on (2024) 29:484 viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Iketani, Liu, Guo, Liu, Chan et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature, doi:10.1038/s41586-022-04594-4
Kang, Kim, Kim, Lim, Jang et al., Virological characteristics and the rapid antigen test as deisolation criteria in immunocompromised patients with COVID-19: a prospective cohort study, J Med Virol, doi:10.1002/jmv.29228
Machkovech, Hahn, Wang, Grubaugh, Halfmann et al., Persistent SARS-CoV-2 infection: significance and implications, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00815-0
Maraolo, Moriello, Gentile, Re: Persistent COVID-19 in immunocompromised patients-Israeli Society of Infectious Diseases consensus statement on diagnosis and management by Meijer et al, Clin Microbiol Infect, doi:10.1016/j.cmi.2024.05.011
Mazzitelli, Mengato, Sasset, Ferrari, Gardin et al., Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study, Viruses, doi:10.3390/v15020384
Mazzitelli, Trunfio, Sasset, Scaglione, Ferrari et al., Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study, J Med Virol, doi:10.1002/jmv.28660
Mccaffrey, Ridgeway, Morral, Propensity score estimation with boosted regression for evaluating causal effects in observational studies, Psychol Methods, doi:10.1037/1082-989X.9.4.403
Meijer, Paran, Belkin, Brosh-Nissimov, Persistent COVID-19 in immunocompromised patients-Israeli society of infectious diseases consensus statement on diagnosis and management'-Author's reply, Clin Microbiol Infect, doi:10.1016/j.cmi.2024.05.022
Mengato, Mazzitelli, Francavilla, Bettio, Sasset et al., Changing patterns and clinical outcomes of hospitalized patients with COVID-19 severe pneumonia treated with remdesivir according to vaccination status: results from a real-world retrospective study, Clin Exp Med, doi:10.1007/s10238-023-01036-x
Mikulska, Sepulcri, Dentone, Magne, Balletto et al., Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients, Clin Infect Dis, doi:10.1093/cid/ciad181
Mozaffari, Chandak, Gottlieb, Chima-Melton, Read et al., Remdesivir reduced mortality in immunocompromised patients hospitalized for COVID-19 across variant waves: findings from routine clinical practice, Clin Infect Dis, doi:10.1093/cid/ciad460
Orth, Flasshove, Berger, Hattenhauer, Biederbick et al., Early combination therapy of COVID-19 in highrisk patients, Infection, doi:10.1007/s15010-023-02125-5
Razonable, Protecting the vulnerable: addressing the COVID-19 care needs of people with compromised immunity, Front Immunol, doi:10.3389/fimmu.2024.1397040
Rotundo, Berardelli, Gulli, Gamba, Russo, Early initiation of combined therapy in severely immunocompromised patients with COVID-19: a retrospective cohort study, BMC Infect Dis, doi:10.1186/s12879-024-09466-y
Scaglione, Rotundo, Marascio, Marco, Veneziano, Publisher Correction: Lessons learned and implications of early therapies for coronavirus disease in a territorial service centre in the Calabria region: a retrospective study, BMC Infect Dis, doi:10.1186/s12879-022-07871-9
Smith, Lambrou, Patel, SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7251a1
Spaccarotella, Mazzitelli, Migliarino, Curcio, Rosa et al., Therapy with RAS inhibitors during the COVID-19 pandemic, J Cardiovasc Med, doi:10.2459/JCM.0000000000001160
Stanleyraj, Sethuraman, Gupta, Thiruvoth, Gupta et al., Treating COVID-19: are we missing out the window of opportunity?, J Antimicrob Chemother, doi:10.1093/jac/dkaa442
Torti, Olimpieri, Bonfanti, Tascini, Celant et al., Real-life comparison of mortality in patients with SARS-CoV-2 infection at risk for clinical progression treated with molnupiravir or nirmatrelvir plus ritonavir during the Omicron era in Italy: a nationwide, cohort study, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2023.100684
Vito, Colpani, Poliseno, Diella, Ieva et al., What is the efficacy of sotrovimab in reducing disease progression and death in people with COVID-19 during the Omicron Era? Answers from a real-life study, Viruses, doi:10.3390/v15081757
Vito, Moi, Saderi, Puci, Colpani et al., Vaccination and antiviral treatment reduce the time to negative SARS-CoV-2 swab: a real-life study, Viruses, doi:10.3390/v15112180
Vito, Saderi, Colpani, Puci, Zauli et al., New score to predict COVID-19 progression in vaccine and early treatment era: the COVID-19 Sardinian Progression Score (CSPS), Eur J Med Res, doi:10.1186/s40001-024-01718-6
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
DOI record:
{
"DOI": "10.1186/s40001-024-02062-5",
"ISSN": [
"2047-783X"
],
"URL": "http://dx.doi.org/10.1186/s40001-024-02062-5",
"alternative-id": [
"2062"
],
"article-number": "484",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "3 August 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 September 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "4 October 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Study protocol was approved by Local Ethic Committee (n. AOP 0002323, January 1rst, 2022). Each patient was requested to sign written informed consent for participation."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "M.M. and A.C. received a research grant from Gilead Sciences, speakers’ honoraria from ViiV Healthcare, Gilead Sciences, and Merck Sharp & Dohme, and advisory board fees from ViiV Healthcare. V.S. received speakers’ honoraria from Angelini. All the other authors do not have any conflicts of interest to disclose. The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0263-0703",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maria",
"given": "Mazzitelli",
"sequence": "first"
},
{
"affiliation": [],
"family": "Maraolo",
"given": "Alberto Enrico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cozzolino",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sasset",
"given": "Lolita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrari",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Basso",
"given": "Monica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vania",
"given": "Eleonora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonadiman",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scaglione",
"given": "Vincenzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cattelan",
"given": "Anna Maria",
"sequence": "additional"
}
],
"container-title": "European Journal of Medical Research",
"container-title-short": "Eur J Med Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
10,
4
]
],
"date-time": "2024-10-04T07:02:08Z",
"timestamp": 1728025328000
},
"deposited": {
"date-parts": [
[
2024,
10,
4
]
],
"date-time": "2024-10-04T07:05:19Z",
"timestamp": 1728025519000
},
"indexed": {
"date-parts": [
[
2024,
10,
5
]
],
"date-time": "2024-10-05T04:07:39Z",
"timestamp": 1728101259916
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
10,
4
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
4
]
],
"date-time": "2024-10-04T00:00:00Z",
"timestamp": 1728000000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
4
]
],
"date-time": "2024-10-04T00:00:00Z",
"timestamp": 1728000000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40001-024-02062-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40001-024-02062-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40001-024-02062-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
10,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
4
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3389/fimmu.2024.1397040",
"author": "RR Razonable",
"doi-asserted-by": "publisher",
"first-page": "1397040",
"journal-title": "Front Immunol",
"key": "2062_CR1",
"unstructured": "Razonable RR. Protecting the vulnerable: addressing the COVID-19 care needs of people with compromised immunity. Front Immunol. 2024;15:1397040. https://doi.org/10.3389/fimmu.2024.1397040.",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciad546",
"author": "S Feys",
"doi-asserted-by": "publisher",
"first-page": "361",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "2062_CR2",
"unstructured": "Feys S, Lagrou K, Lauwers HM, Haenen K, Jacobs C, Brusselmans M, et al. High burden of COVID-19-associated pulmonary aspergillosis in severely immunocompromised patients requiring mechanical ventilation. Clin Infect Dis. 2024;78(2):361–70. https://doi.org/10.1093/cid/ciad546.",
"volume": "78",
"year": "2024"
},
{
"DOI": "10.1016/j.immuni.2022.09.006",
"author": "S DeWolf",
"doi-asserted-by": "publisher",
"first-page": "1779",
"issue": "10",
"journal-title": "Immunity",
"key": "2062_CR3",
"unstructured": "DeWolf S, Laracy JC, Perales MA, Kamboj M, van den Brink MRM, Vardhana S. SARS-CoV-2 in immunocompromised individuals. Immunity. 2022;55(10):1779–98. https://doi.org/10.1016/j.immuni.2022.09.006.",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1186/s40001-024-01718-6",
"author": "A De Vito",
"doi-asserted-by": "publisher",
"first-page": "123",
"issue": "1",
"journal-title": "Eur J Med Res",
"key": "2062_CR4",
"unstructured": "De Vito A, Saderi L, Colpani A, Puci MV, Zauli B, Fiore V, et al. New score to predict COVID-19 progression in vaccine and early treatment era: the COVID-19 Sardinian Progression Score (CSPS). Eur J Med Res. 2024;29(1):123. https://doi.org/10.1186/s40001-024-01718-6.",
"volume": "29",
"year": "2024"
},
{
"DOI": "10.1038/s41579-024-01036-y",
"author": "JF Chan",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Microbiol",
"key": "2062_CR5",
"unstructured": "Chan JF, Yuan S, Chu H, Sridhar S, Yuen KY. COVID-19 drug discovery and treatment options. Nat Rev Microbiol. 2024. https://doi.org/10.1038/s41579-024-01036-y.",
"year": "2024"
},
{
"DOI": "10.3389/fmed.2021.639970",
"author": "A Di Castelnuovo",
"doi-asserted-by": "publisher",
"first-page": "639970",
"journal-title": "Front Med (Lausanne)",
"key": "2062_CR6",
"unstructured": "Di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, et al. Lopinavir/ritonavir and darunavir/cobicistat in hospitalized COVID-19 patients: findings from the multicenter Italian CORIST study. Front Med (Lausanne). 2021;8:639970. https://doi.org/10.3389/fmed.2021.639970.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.3390/v15112180",
"author": "A De Vito",
"doi-asserted-by": "publisher",
"first-page": "2180",
"issue": "11",
"journal-title": "Viruses",
"key": "2062_CR7",
"unstructured": "De Vito A, Moi G, Saderi L, Puci MV, Colpani A, Firino L, et al. Vaccination and antiviral treatment reduce the time to negative SARS-CoV-2 swab: a real-life study. Viruses. 2023;15(11):2180. https://doi.org/10.3390/v15112180.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.2459/JCM.0000000000001160",
"author": "C Spaccarotella",
"doi-asserted-by": "publisher",
"first-page": "329",
"issue": "5",
"journal-title": "J Cardiovasc Med (Hagerstown)",
"key": "2062_CR8",
"unstructured": "Spaccarotella C, Mazzitelli M, Migliarino S, Curcio A, De Rosa S, Torti C, et al. Therapy with RAS inhibitors during the COVID-19 pandemic. J Cardiovasc Med (Hagerstown). 2021;22(5):329–34. https://doi.org/10.2459/JCM.0000000000001160.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1007/s10238-023-01036-x",
"author": "D Mengato",
"doi-asserted-by": "publisher",
"first-page": "2749",
"issue": "6",
"journal-title": "Clin Exp Med",
"key": "2062_CR9",
"unstructured": "Mengato D, Mazzitelli M, Francavilla A, Bettio M, Sasset L, Presa N, et al. Changing patterns and clinical outcomes of hospitalized patients with COVID-19 severe pneumonia treated with remdesivir according to vaccination status: results from a real-world retrospective study. Clin Exp Med. 2023;23(6):2749–56. https://doi.org/10.1007/s10238-023-01036-x.",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.3390/v15020384",
"author": "M Mazzitelli",
"doi-asserted-by": "publisher",
"first-page": "384",
"issue": "2",
"journal-title": "Viruses",
"key": "2062_CR10",
"unstructured": "Mazzitelli M, Mengato D, Sasset L, Ferrari A, Gardin S, Scaglione V, et al. Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study. Viruses. 2023;15(2):384. https://doi.org/10.3390/v15020384.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1007/s40472-022-00385-y",
"author": "V Dioverti",
"doi-asserted-by": "publisher",
"first-page": "209",
"issue": "4",
"journal-title": "Curr Transplant Rep",
"key": "2062_CR11",
"unstructured": "Dioverti V, Salto-Alejandre S, Haidar G. Immunocompromised patients with protracted COVID-19: a Review of “Long Persisters.” Curr Transplant Rep. 2022;9(4):209–18. https://doi.org/10.1007/s40472-022-00385-y.",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1186/s12985-023-02269-8",
"author": "I Gentile",
"doi-asserted-by": "publisher",
"first-page": "301",
"issue": "1",
"journal-title": "Virol J",
"key": "2062_CR12",
"unstructured": "Gentile I, Foggia M, Silvitelli M, Sardanelli A, Cattaneo L, Viceconte G. Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab. Virol J. 2023;20(1):301. https://doi.org/10.1186/s12985-023-02269-8.",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1128/cmr.00119-23",
"author": "D Focosi",
"doi-asserted-by": "publisher",
"first-page": "e0011923",
"journal-title": "Clin Microbiol Rev",
"key": "2062_CR13",
"unstructured": "Focosi D, Franchini M, Maggi F, Shoham S. COVID-19 therapeutics. Clin Microbiol Rev. 2024;2024:e0011923. https://doi.org/10.1128/cmr.00119-23.",
"volume": "2024",
"year": "2024"
},
{
"DOI": "10.1016/j.lanepe.2023.100684",
"author": "C Torti",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Eur",
"key": "2062_CR14",
"unstructured": "Torti C, Olimpieri PP, Bonfanti P, Tascini C, Celant S, Tacconi D, et al. Real-life comparison of mortality in patients with SARS-CoV-2 infection at risk for clinical progression treated with molnupiravir or nirmatrelvir plus ritonavir during the Omicron era in Italy: a nationwide, cohort study. Lancet Reg Health Eur. 2023;31: 100684. https://doi.org/10.1016/j.lanepe.2023.100684.",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1021/acscentsci.0c00489",
"author": "RT Eastman",
"doi-asserted-by": "publisher",
"first-page": "672",
"issue": "5",
"journal-title": "ACS Cent Sci",
"key": "2062_CR15",
"unstructured": "Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–83. https://doi.org/10.1021/acscentsci.0c00489.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1007/s40121-023-00900-3",
"author": "PO Godwin",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Infect Dis Ther",
"key": "2062_CR16",
"unstructured": "Godwin PO, Polsonetti B, Caron MF, Oppelt TF. Remdesivir for the treatment of COVID-19: a narrative review. Infect Dis Ther. 2024;13(1):1–19. https://doi.org/10.1007/s40121-023-00900-3.",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciad460",
"author": "E Mozaffari",
"doi-asserted-by": "publisher",
"first-page": "1626",
"issue": "12",
"journal-title": "Clin Infect Dis",
"key": "2062_CR17",
"unstructured": "Mozaffari E, Chandak A, Gottlieb RL, Chima-Melton C, Read SH, Jiang H, et al. Remdesivir reduced mortality in immunocompromised patients hospitalized for COVID-19 across variant waves: findings from routine clinical practice. Clin Infect Dis. 2023;77(12):1626–34. https://doi.org/10.1093/cid/ciad460.",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28660",
"author": "M Mazzitelli",
"doi-asserted-by": "publisher",
"first-page": "e28660",
"issue": "3",
"journal-title": "J Med Virol",
"key": "2062_CR18",
"unstructured": "Mazzitelli M, Trunfio M, Sasset L, Scaglione V, Ferrari A, Mengato D, et al. Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study. J Med Virol. 2023;95(3):e28660. https://doi.org/10.1002/jmv.28660.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29082",
"author": "JM Caso",
"doi-asserted-by": "publisher",
"issue": "9",
"journal-title": "J Med Virol",
"key": "2062_CR19",
"unstructured": "Caso JM, Fernandez-Ruiz M, Lopez-Medrano F, Caro-Teller JM, Lizasoain M, San-Juan R, et al. Nirmatrelvir/ritonavir for the treatment of immunocompromised adult patients with early-stage symptomatic COVID-19: a real-life experience. J Med Virol. 2023;95(9): e29082. https://doi.org/10.1002/jmv.29082.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3390/v15071515",
"author": "D Basoulis",
"doi-asserted-by": "publisher",
"first-page": "1515",
"issue": "7",
"journal-title": "Viruses",
"key": "2062_CR20",
"unstructured": "Basoulis D, Tsakanikas A, Gkoufa A, Bitsani A, Karamanakos G, Mastrogianni E, et al. Effectiveness of oral nirmatrelvir/ritonavir vs. intravenous three-day remdesivir in preventing progression to severe COVID-19: a single-center, prospective, comparative, real-life study. Viruses. 2023;15(7):1515. https://doi.org/10.3390/v15071515.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1007/s40121-024-00994-3",
"author": "F Bai",
"doi-asserted-by": "publisher",
"first-page": "1589",
"issue": "7",
"journal-title": "Infect Dis Ther",
"key": "2062_CR21",
"unstructured": "Bai F, Beringheli T, Vitaletti V, Santoro A, Mola F, Copes A, et al. Clinical outcome and 7-day virological clearance in high-risk patients with mild-moderate COVID-19 treated with molnupiravir, nirmatrelvir/ritonavir, or remdesivir. Infect Dis Ther. 2024;13(7):1589–605. https://doi.org/10.1007/s40121-024-00994-3.",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1001/jama.2021.0202",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "632",
"issue": "7",
"journal-title": "JAMA",
"key": "2062_CR22",
"unstructured": "Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–44. https://doi.org/10.1001/jama.2021.0202.",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "2062_CR23",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385(23): e81. https://doi.org/10.1056/NEJMoa2108163.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"author": "S Iketani",
"doi-asserted-by": "publisher",
"first-page": "553",
"issue": "7906",
"journal-title": "Nature",
"key": "2062_CR24",
"unstructured": "Iketani S, Liu L, Guo Y, Liu L, Chan JF, Huang Y, et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604(7906):553–6. https://doi.org/10.1038/s41586-022-04594-4.",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciad181",
"author": "M Mikulska",
"doi-asserted-by": "publisher",
"first-page": "280",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "2062_CR25",
"unstructured": "Mikulska M, Sepulcri C, Dentone C, Magne F, Balletto E, Baldi F, et al. Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients. Clin Infect Dis. 2023;77(2):280–6. https://doi.org/10.1093/cid/ciad181.",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1126/science.abm3425",
"author": "PB Gilbert",
"doi-asserted-by": "publisher",
"first-page": "43",
"issue": "6576",
"journal-title": "Science",
"key": "2062_CR26",
"unstructured": "Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. https://doi.org/10.1126/science.abm3425.",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiad181",
"author": "A Antinori",
"doi-asserted-by": "publisher",
"first-page": "S4",
"issue": "Suppl 1",
"journal-title": "J Infect Dis",
"key": "2062_CR27",
"unstructured": "Antinori A, Bausch-Jurken M. The burden of COVID-19 in the immunocompromised patient: implications for vaccination and needs for the future. J Infect Dis. 2023;228(Suppl 1):S4–12. https://doi.org/10.1093/infdis/jiad181.",
"volume": "228",
"year": "2023"
},
{
"DOI": "10.1136/bmj.p2622",
"doi-asserted-by": "publisher",
"key": "2062_CR28",
"unstructured": "Update to living WHO guideline on drugs for covid-19. BMJ. 2023;383:2622. https://doi.org/10.1136/bmj.p2622."
},
{
"DOI": "10.1093/aje/kwg115",
"author": "MS Cepeda",
"doi-asserted-by": "publisher",
"first-page": "280",
"issue": "3",
"journal-title": "Am J Epidemiol",
"key": "2062_CR29",
"unstructured": "Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158(3):280–7. https://doi.org/10.1093/aje/kwg115.",
"volume": "158",
"year": "2003"
},
{
"DOI": "10.1093/ckj/sfab158",
"author": "NC Chesnaye",
"doi-asserted-by": "publisher",
"first-page": "14",
"issue": "1",
"journal-title": "Clin Kidney J",
"key": "2062_CR30",
"unstructured": "Chesnaye NC, Stel VS, Tripepi G, Dekker FW, Fu EL, Zoccali C, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15(1):14–20. https://doi.org/10.1093/ckj/sfab158.",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1037/1082-989X.9.4.403",
"author": "DF McCaffrey",
"doi-asserted-by": "publisher",
"first-page": "403",
"issue": "4",
"journal-title": "Psychol Methods",
"key": "2062_CR31",
"unstructured": "McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–25. https://doi.org/10.1037/1082-989X.9.4.403.",
"volume": "9",
"year": "2004"
},
{
"DOI": "10.1093/aje/kwj149",
"author": "MA Brookhart",
"doi-asserted-by": "publisher",
"first-page": "1149",
"issue": "12",
"journal-title": "Am J Epidemiol",
"key": "2062_CR32",
"unstructured": "Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–56. https://doi.org/10.1093/aje/kwj149.",
"volume": "163",
"year": "2006"
},
{
"DOI": "10.1016/j.jpainsymman.2014.05.014",
"author": "MM Garrido",
"doi-asserted-by": "publisher",
"first-page": "711",
"issue": "4",
"journal-title": "J Pain Symptom Manage",
"key": "2062_CR33",
"unstructured": "Garrido MM. Propensity scores: a practical method for assessing treatment effects in pain and symptom management research. J Pain Symptom Manage. 2014;48(4):711–8. https://doi.org/10.1016/j.jpainsymman.2014.05.014.",
"volume": "48",
"year": "2014"
},
{
"DOI": "10.1007/s15010-023-02125-5",
"author": "HM Orth",
"doi-asserted-by": "publisher",
"first-page": "877",
"issue": "3",
"journal-title": "Infection",
"key": "2062_CR34",
"unstructured": "Orth HM, Flasshove C, Berger M, Hattenhauer T, Biederbick KD, Mispelbaum R, et al. Early combination therapy of COVID-19 in high-risk patients. Infection. 2024;52(3):877–89. https://doi.org/10.1007/s15010-023-02125-5.",
"volume": "52",
"year": "2024"
},
{
"DOI": "10.1093/cid/ciac724",
"author": "A Bhimraj",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "2062_CR35",
"unstructured": "Bhimraj A, Morgan RL, Shumaker AH, Baden L, Cheng VCC, Edwards KM, et al. Infectious diseases society of America Guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2022. https://doi.org/10.1093/cid/ciac724.",
"year": "2022"
},
{
"DOI": "10.1186/s12879-022-07871-9",
"author": "V Scaglione",
"doi-asserted-by": "publisher",
"first-page": "883",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "2062_CR36",
"unstructured": "Scaglione V, Rotundo S, Marascio N, De Marco C, Lionello R, Veneziano C, et al. Publisher Correction: Lessons learned and implications of early therapies for coronavirus disease in a territorial service centre in the Calabria region: a retrospective study. BMC Infect Dis. 2022;22(1):883. https://doi.org/10.1186/s12879-022-07871-9.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1186/s12879-024-09466-y",
"author": "S Rotundo",
"doi-asserted-by": "publisher",
"first-page": "564",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "2062_CR37",
"unstructured": "Rotundo S, Berardelli L, Gulli S, La Gamba V, Lionello R, Russo A, et al. Early initiation of combined therapy in severely immunocompromised patients with COVID-19: a retrospective cohort study. BMC Infect Dis. 2024;24(1):564. https://doi.org/10.1186/s12879-024-09466-y.",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1016/j.lanepe.2023.100747",
"author": "RA Evans",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Eur",
"key": "2062_CR38",
"unstructured": "Evans RA, Dube S, Lu Y, Yates M, Arnetorp S, Barnes E, et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study. Lancet Reg Health Eur. 2023;35: 100747. https://doi.org/10.1016/j.lanepe.2023.100747.",
"volume": "35",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciae308",
"author": "M Boeckh",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "2062_CR39",
"unstructured": "Boeckh M, Pergam SA, Limaye AP, Englund J, Corey L, Hill JA. How immunocompromised hosts were left behind in the quest to control the Covid-19 Pandemic. Clin Infect Dis. 2024. https://doi.org/10.1093/cid/ciae308.",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(23)00815-0",
"author": "HM Machkovech",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "2062_CR40",
"unstructured": "Machkovech HM, Hahn AM, Garonzik Wang J, Grubaugh ND, Halfmann PJ, Johnson MC, et al. Persistent SARS-CoV-2 infection: significance and implications. Lancet Infect Dis. 2024. https://doi.org/10.1016/S1473-3099(23)00815-0.",
"year": "2024"
},
{
"DOI": "10.1016/j.cmi.2024.05.022",
"author": "SE Meijer",
"doi-asserted-by": "publisher",
"journal-title": "Clin Microbiol Infect",
"key": "2062_CR41",
"unstructured": "Meijer SE, Paran Y, Belkin A, Brosh-Nissimov T. ’Persistent COVID-19 in immunocompromised patients—Israeli society of infectious diseases consensus statement on diagnosis and management’—Author’s reply. Clin Microbiol Infect. 2024. https://doi.org/10.1016/j.cmi.2024.05.022.",
"year": "2024"
},
{
"DOI": "10.1016/j.cmi.2024.05.011",
"author": "AE Maraolo",
"doi-asserted-by": "publisher",
"journal-title": "Clin Microbiol Infect",
"key": "2062_CR42",
"unstructured": "Maraolo AE, Moriello NS, Gentile I. Re: Persistent COVID-19 in immunocompromised patients—Israeli Society of Infectious Diseases consensus statement on diagnosis and management by Meijer et al. Clin Microbiol Infect. 2024. https://doi.org/10.1016/j.cmi.2024.05.011.",
"year": "2024"
},
{
"DOI": "10.1016/j.ijid.2023.09.021",
"author": "D Focosi",
"doi-asserted-by": "publisher",
"first-page": "55",
"journal-title": "Int J Infect Dis",
"key": "2062_CR43",
"unstructured": "Focosi D, Maggi F, D’Abramo A, Nicastri E, Sullivan DJ. Antiviral combination therapies for persistent COVID-19 in immunocompromised patients. Int J Infect Dis. 2023;137:55–9. https://doi.org/10.1016/j.ijid.2023.09.021.",
"volume": "137",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkaa442",
"author": "J Sundararaj Stanleyraj",
"doi-asserted-by": "publisher",
"first-page": "283",
"issue": "2",
"journal-title": "J Antimicrob Chemother",
"key": "2062_CR44",
"unstructured": "Sundararaj Stanleyraj J, Sethuraman N, Gupta R, Thiruvoth S, Gupta M, Ryo A. Treating COVID-19: are we missing out the window of opportunity? J Antimicrob Chemother. 2021;76(2):283–5. https://doi.org/10.1093/jac/dkaa442.",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"author": "M Cevik",
"doi-asserted-by": "publisher",
"first-page": "e13",
"issue": "1",
"journal-title": "Lancet Microbe",
"key": "2062_CR45",
"unstructured": "Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–22. https://doi.org/10.1016/S2666-5247(20)30172-5.",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.3390/v16020217",
"author": "D Focosi",
"doi-asserted-by": "publisher",
"first-page": "217",
"issue": "2",
"journal-title": "Viruses",
"key": "2062_CR46",
"unstructured": "Focosi D, Casadevall A, Franchini M, Maggi F. Sotrovimab: a review of its efficacy against SARS-CoV-2 variants. Viruses. 2024;16(2):217. https://doi.org/10.3390/v16020217.",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.3390/v15081757",
"author": "A De Vito",
"doi-asserted-by": "publisher",
"first-page": "1757",
"issue": "8",
"journal-title": "Viruses",
"key": "2062_CR47",
"unstructured": "De Vito A, Colpani A, Poliseno M, Diella L, Ieva FRP, Belati A, et al. What is the efficacy of sotrovimab in reducing disease progression and death in people with COVID-19 during the Omicron Era? Answers from a real-life study. Viruses. 2023;15(8):1757. https://doi.org/10.3390/v15081757.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7251a1",
"author": "DJ Smith",
"doi-asserted-by": "publisher",
"first-page": "1357",
"issue": "51",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2062_CR48",
"unstructured": "Smith DJ, Lambrou A, Patel P. SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals. MMWR Morb Mortal Wkly Rep. 2023;72(51):1357–64. https://doi.org/10.15585/mmwr.mm7251a1.",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29228",
"author": "SW Kang",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "J Med Virol",
"key": "2062_CR49",
"unstructured": "Kang SW, Kim JW, Kim JY, Lim SY, Jang CY, Chang E, et al. Virological characteristics and the rapid antigen test as deisolation criteria in immunocompromised patients with COVID-19: a prospective cohort study. J Med Virol. 2023;95(11): e29228. https://doi.org/10.1002/jmv.29228.",
"volume": "95",
"year": "2023"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://eurjmedres.biomedcentral.com/articles/10.1186/s40001-024-02062-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Does early combination vs. Monotherapy improve clinical outcomes of clinically extremely vulnerable patients with COVID-19? Results from a retrospective propensity-weighted analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "29"
}
