A potential harmful effect of dexamethasone in non-severe COVID-19: results from the COPPER-pilot study
et al., ERJ Open Research, doi:10.1183/23120541.00129-2022, COPPER, NCT04746430, Apr 2022
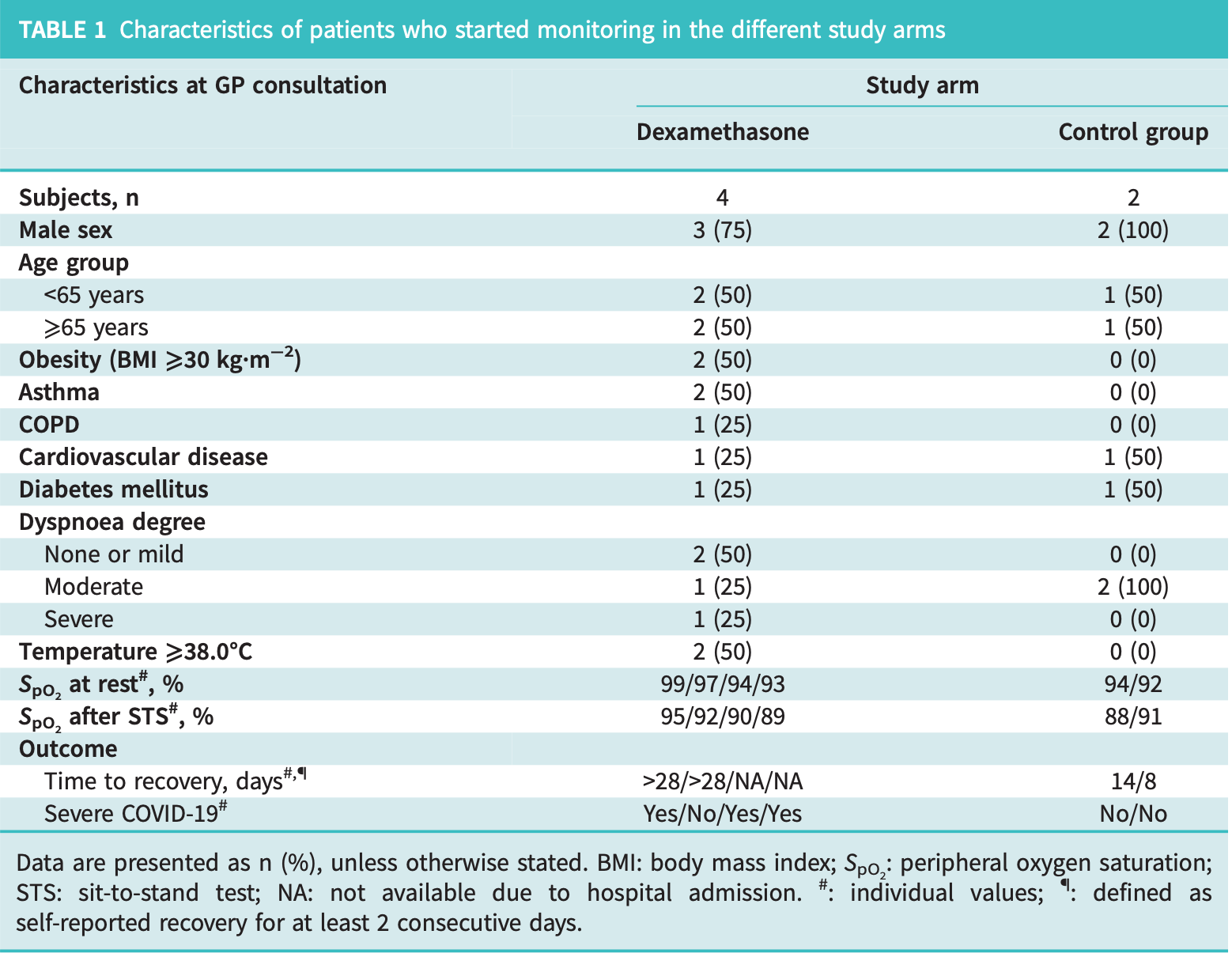
Pilot RCT of 7 outpatients with non-severe COVID-19 suggesting potential harmful effects of dexamethasone treatment. Time to recovery was significantly longer in the dexamethasone group compared to controls (p=0.03). Authors note that systemic corticosteroids, while beneficial for hospitalized COVID-19 patients requiring oxygen, may be harmful in non-severe cases by potentially inhibiting normal immune response when administered too early.
|
risk of hospitalization, 300.0% higher, RR 4.00, p = 0.47, treatment 2 of 4 (50.0%), control 0 of 2 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of severe case, 450.0% higher, RR 5.50, p = 0.40, treatment 3 of 4 (75.0%), control 0 of 2 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 600.0% higher, RR 7.00, p = 0.07, treatment 4 of 4 (100.0%), control 0 of 2 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kocks et al., 30 Apr 2022, Double Blind Randomized Controlled Trial, Netherlands, peer-reviewed, 9 authors, trial NCT04746430 (history) (COPPER).
Contact: permissions@ersnet.org, janwillem@gpri.nl.
doi:10.1183/23120541.00129-2022].
A potential harmful effect of dexamethasone in non-severe COVID-19: results from the COPPER-pilot study To the Editor: The coronavirus disease 2019 (COVID-19) pandemic poses major challenges to healthcare professionals. General practitioners (GPs) are at the frontline and may play an important role in preventing progression to severe disease, and in countering shortages of hospital beds. However, guideline-based treatment options for COVID-19 are still limited for GPs [1] . Systemic administration of corticosteroids has been demonstrated to improve survival of hospitalised patients with COVID-19 who require oxygen supplementation therapy, but harm in hospitalised patients on room air could not be excluded [2]. Inhaled corticosteroids have been shown to reduce time to recovery in two open-label randomised controlled trials (RCTs) of inhaled budesonide [3, 4]. However, two double-blind RCTs of inhaled ciclesonide could not confirm [5, 6] . Administration of systemic corticosteroids to out-of-hospital patients who show mild to moderate pulmonary symptoms with signs of desaturation can be hypothesised to prevent progression to severe disease and perhaps alleviate strains on hospitals during the pandemic. However, while preventing the progression to overwhelming inflammation and cytokine-related lung injury on the one hand, steroids may also inhibit the normal immune response when administered too early on the other hand. There are currently no data available to support the use of oral corticosteroids to treat patients with deteriorating COVID-19 by GPs. We designed an open-label RCT studying the effectiveness and safety of treatment with dexamethasone in preventing the development of severe COVID-19 requiring hospitalisation, for which we first started a pilot phase (ClinicalTrials.gov identifier number:
Conflict of interest: J. Kocks, M. Kerkhof, A. van de Maat, I. van Geer-Postmus and T. le Rütte were employed by the General Practitioners Research Institute (GPRI) at the time of the study. In the past 3 years (2019-2022), the GPRI conducted investigator-and sponsor-initiated research funded by noncommercial organisations, academic institutes and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis and Teva). H. Kerstjens reports no conflicts of interest for this study; unrelated to this study, his institution has received funding for his studies and payments for his consultancy from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis. R. Gans, J. Scherpenisse and J. Schaart report no conflicts of interest for this study. Support statement: This work was supported by General Practitioner Care Drenthe, the Netherlands. Funding information for this article has been deposited with the Crossref Funder Registry.
References
Briand, Behal, Chenivesse, The 1-minute sit-to-stand test to detect exercise-induced oxygen desaturation in patients with interstitial lung disease, Ther Adv Respir Dis
Chen, Yin, Tan, Effectiveness of systemic corticosteroids therapy for nonsevere patients with COVID-19: a multicenter, retrospective, longitudinal cohort study, Value Health
Clemency, Varughese, Gonzalez-Rojas, Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial, JAMA Intern Med
Ezer, Belga, Daneman, Inhaled and intranasal ciclesonide for the treatment of COVID-19 in adult outpatients: CONTAIN phase II randomised controlled trial, BMJ
Li, Li, Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study, Infect Dis Ther
Ramakrishnan, Nicolau, Langford, Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med
Shuto, Komiya, Yamasue, A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19, Sci Rep
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA
Vita, Centanni, Lanini, Benefits of steroid therapy in COVID-19 patients with different PaO 2 /FiO 2 ratio at admission, J Clin Med
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1183/23120541.00129-2022
DOI record:
{
"DOI": "10.1183/23120541.00129-2022",
"ISSN": [
"2312-0541"
],
"URL": "http://dx.doi.org/10.1183/23120541.00129-2022",
"accepted": {
"date-parts": [
[
2022,
5,
2
]
]
},
"alternative-id": [
"10.1183/23120541.00129-2022"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-2760-0693",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kocks",
"given": "Janwillem",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-3823-1061",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kerkhof",
"given": "Marjan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scherpenisse",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van de Maat",
"given": "Aimée",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Geer-Postmus",
"given": "Iris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "le Rütte",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaart",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gans",
"given": "Reinold O.B.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7705-7927",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kerstjens",
"given": "Huib A.M.",
"sequence": "additional"
}
],
"container-title": "ERJ Open Research",
"container-title-short": "ERJ Open Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"publications.ersnet.org"
]
},
"created": {
"date-parts": [
[
2022,
5,
23
]
],
"date-time": "2022-05-23T16:09:16Z",
"timestamp": 1653322156000
},
"deposited": {
"date-parts": [
[
2025,
2,
24
]
],
"date-time": "2025-02-24T05:18:54Z",
"timestamp": 1740374334000
},
"funder": [
{
"award": [
"not applicable"
],
"name": "General Practitioner Care Drenthe, the Netherlands"
}
],
"indexed": {
"date-parts": [
[
2025,
3,
19
]
],
"date-time": "2025-03-19T14:39:46Z",
"timestamp": 1742395186328,
"version": "3.37.3"
},
"is-referenced-by-count": 14,
"issue": "2",
"issued": {
"date-parts": [
[
2022,
4
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2022,
5,
30
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T00:00:00Z",
"timestamp": 1648771200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/23120541.00129-2022",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "00129-2022",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2022,
4
]
]
},
"published-online": {
"date-parts": [
[
2022,
5,
13
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"key": "2024101711353777000_8.2.00129-2022.1",
"unstructured": "World Health Organization. Corticosteroids for COVID-19: living guidance. Date last accessed: 21 April 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2024101711353777000_8.2.00129-2022.2"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"doi-asserted-by": "publisher",
"key": "2024101711353777000_8.2.00129-2022.3"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"author": "Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Lancet Respir Med",
"key": "2024101711353777000_8.2.00129-2022.4",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.6759",
"article-title": "Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial",
"author": "Clemency",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "JAMA Intern Med",
"key": "2024101711353777000_8.2.00129-2022.5",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2021-068060",
"doi-asserted-by": "publisher",
"key": "2024101711353777000_8.2.00129-2022.6"
},
{
"DOI": "10.1007/s40121-020-00332-3",
"article-title": "Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "823",
"journal-title": "Infect Dis Ther",
"key": "2024101711353777000_8.2.00129-2022.7",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-78054-2",
"doi-asserted-by": "publisher",
"key": "2024101711353777000_8.2.00129-2022.8"
},
{
"DOI": "10.1177/1753466618793028",
"article-title": "The 1-minute sit-to-stand test to detect exercise-induced oxygen desaturation in patients with interstitial lung disease",
"author": "Briand",
"doi-asserted-by": "crossref",
"first-page": "1753466618793028",
"journal-title": "Ther Adv Respir Dis",
"key": "2024101711353777000_8.2.00129-2022.9",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "2024101711353777000_8.2.00129-2022.10"
},
{
"DOI": "10.1016/j.jval.2021.12.013",
"article-title": "Effectiveness of systemic corticosteroids therapy for nonsevere patients with COVID-19: a multicenter, retrospective, longitudinal cohort study",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "709",
"journal-title": "Value Health",
"key": "2024101711353777000_8.2.00129-2022.11",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.3390/jcm10153236",
"article-title": "Benefits of steroid therapy in COVID-19 patients with different PaO2/FiO2 ratio at admission",
"author": "Vita",
"doi-asserted-by": "crossref",
"first-page": "3236",
"journal-title": "J Clin Med",
"key": "2024101711353777000_8.2.00129-2022.12",
"volume": "10",
"year": "2021"
},
{
"key": "2024101711353777000_8.2.00129-2022.13",
"unstructured": "COVID-19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda, National Institutes of Health. Date last accessed : 21 April 2022. https://www.covid19treatmentguidelines.nih.gov/"
}
],
"reference-count": 13,
"references-count": 13,
"relation": {},
"resource": {
"primary": {
"URL": "https://publications.ersnet.org/lookup/doi/10.1183/23120541.00129-2022"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A potential harmful effect of dexamethasone in non-severe COVID-19: results from the COPPER-pilot study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1183/ers-crossmark-policy",
"volume": "8"
}