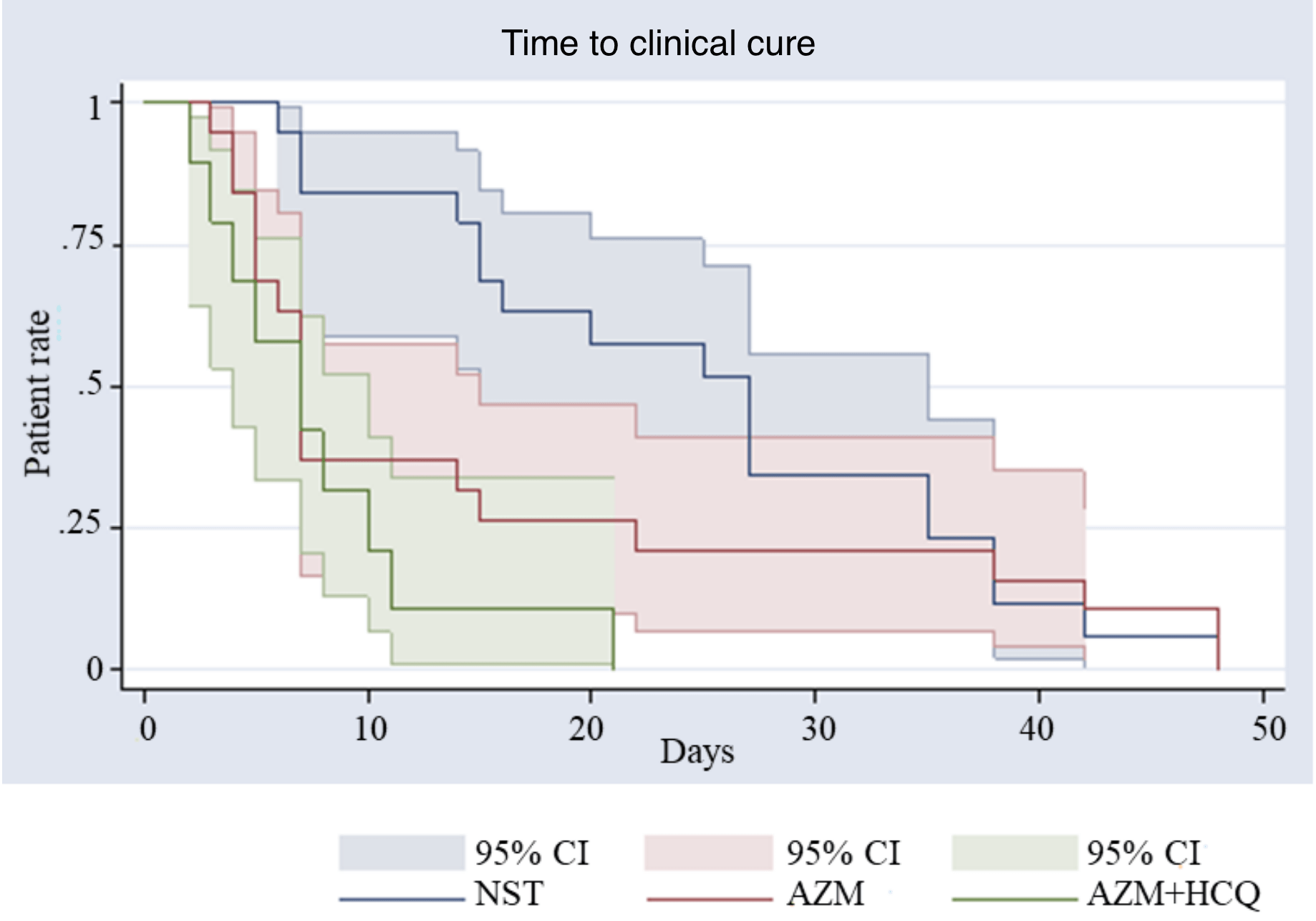
Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19
et al., Asian Journal of Medicine and Health, doi:10.9734/ajmah/2020/v18i730224, May 2020 (preprint)
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Mean clinical recovery time reduced from 26 days (SOC) to 9 days, p<0.0001 (HCQ+AZ) or 13 days, p<0.0001 (AZ). No cardiac toxicity. Small retrospective study of 88 patients with case control analysis with matched patients.
|
risk of death, 61.4% lower, RR 0.39, p = 1.00, treatment 0 of 20 (0.0%), control 1 of 34 (2.9%), NNT 34, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
recovery time, 65.0% lower, relative time 0.35, p < 0.001, treatment 20, control 34.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Guérin et al., 31 May 2020, retrospective, France, peer-reviewed, 8 authors, dosage 600mg days 1-10, 7-10 days, this trial uses multiple treatments in the treatment arm (combined with AZ) - results of individual treatments may vary.
Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19
Asian Journal of Medicine and Health, doi:10.9734/ajmah/2020/v18i730224
Aims: The challenge regarding COVID-19 is to prevent complications and fatal evolution. Azithromycin (AZM) and hydroxychloroquine (HCQ) have proven their antiviral effect in vitro. We aimed to assess the efficacy and safety of AZM alone or combined to HCQ, prescribed, at an early stage, in patients with Covid-19, in a primary care setting. Study Design: Retrospective observational study. Place and Duration of Study: Patients have been followed by private practitioners in France, between March and April 2020. Methodology: Eighty-eight patients received either no or a symptomatic treatment (NST) (n=34) or AZM alone (n=34) or AZM+HCQ (n=20). The efficacy end point was the time to clinical recovery
Authors' contributions This work was carried out in collaboration among all authors. Authors VG and JLT designed the study and wrote the first draft of the manuscript. Authors VG, TL, ES, NRA, P. Lacrosse and MW followed the patients. Author P. Lévy performed the statistical analysis. All authors read and approved the final manuscript.
ETHICAL APPROVAL It is not applicable.
COMPETING INTERESTS Authors have declared that no competing interests exist.
References
Agrawal, Goel, Gupta, Emerging prophylaxis strategies against COVID-19, Monaldi Archives for Chest Disease, doi:10.4081/monaldi.2020.1289
Arshad, Kilgore, Chaudhry, Jacobsen, Wang et al., Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.06.099
Barbosa, Da Silva, Costa, Castro, Razuk-Filho et al., Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed by telemedicine
Chen, Hu, Zhang, Jiang, Han et al., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, Med Rxiv, doi:10.1101/2020.03.22.20040758
Fox, Mechanism of action of hydroxychloroquine as an antirheumatic drug, Semin Arthritis Rheum, doi:10.1016/s0049-0172(10)80012-5
Gao, Tian, Yang, Break through: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends, doi:10.5582/bst.2020.01047
Garcia-Cremades, Solans, Hughes, Ernest, Wallender et al., Optimizing hydroxychloroquine dosing for patients with COVID19: An integrative modeling approach for effective drug repurposing, Clin Pharmacol Ther, doi:10.1002/cpt.1856
Gautret, Lagier, Parola, Hoang, Meddeb et al., Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101663
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open label non-randomized clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105949
Guan, Ni, Hu, Liang, Ou et al., for the China medical treatment expert group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China, New Engl J Med, doi:10.1056/NEJMoa2002032
Keyaerts, Vijgen, Maes, Neyts, Van Ranst, In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2004.08.085
Kim, Jang, Parl, Kim, Hwang et al., Treatment response to hydroxychloroquine, lopinavirritonavir, and antibiotics for moderate COVID-19: A first report on the pharmacological outcomes from South Korea, Med Rxiv, doi:10.1101/2020.05.13.20094193
Lagier, Million, Gautret, Colson, Giraud-Gatineau, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/ azithromycin and other regimens in Marseille, France: A retrospective analysis, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101791
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov, doi:10.1038/s41421-020-0156-0
Mackenzie, Dose refinement in longterm therapy of rheumatoid arthritis with antimalarials, Am J Med, doi:10.1016/0002-9343(83)91269
Menzel, Akbarshahi, Bjermer, Uller, Azithomycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients, Sci Rep, doi:10.1038/srep28698
Meo, Klonoff, Akram, Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19, Eur Rev Med Pharmacol Sci, doi:10.26355/eurrev_202004_21038
Million, Lagier, Gautret, Colson, Fournier et al., Early treatment of 1061 COVID-19 patients with hydroxychloroquine and azithromycin, Marseille, France, Travel Med Infect Dis, doi:.org/10.1016/j.tmaid.2020.101738
Saleh, Gabriels, Chang, Kim, Mansoor et al., The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection, Circ Arrhythm Electrophysiol, doi:10.1161/CIRCEP.120.008662
Savarino, Trani, Donatelli, Cauda, Cassone, New insights into the antiviral effects of chloroquine, Lancet Infect Dis, doi:10.1016/S1473-3099(06)70361-9
Vincent, Bergeron, Benjannet, Erickson, Rollin et al., Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol J, doi:10.1186/1743-422X-2-69
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Yan, Zou, Sun, Li, Xu et al., Anti-malaria drug chloroquine is highly effective in treating avian Influenza A HRN1 virus infection in a animal model, Cell Res, doi:10.1038/cr.2012.165
Yao, Ye, Zhang, Cui, Huang et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
DOI record:
{
"DOI": "10.9734/ajmah/2020/v18i730224",
"ISSN": [
"2456-8414"
],
"URL": "http://dx.doi.org/10.9734/ajmah/2020/v18i730224",
"abstract": "<jats:p>Aims: The challenge regarding COVID-19 is to prevent complications and fatal evolution. Azithromycin (AZM) and hydroxychloroquine (HCQ) have proven their antiviral effect in vitro. We aimed to assess the efficacy and safety of AZM alone or combined to HCQ, prescribed, at an early stage, in patients with Covid-19, in a primary care setting.
\nStudy Design: Retrospective observational study.
\nPlace and Duration of Study: Patients have been followed by private practitioners in France, between March and April 2020.
\nMethodology: Eighty-eight patients received either no or a symptomatic treatment (NST) (n=34) or AZM alone (n=34) or AZM+HCQ (n=20). The efficacy end point was the time to clinical recovery and the safety end point was the occurrence of cardiovascular events. To improve the evidence level, a case-control analysis was performed on a sample of 57 patients (19/group) matched for age, sex and BMI.
\nResults: The mean (SD) times to achieve clinical recovery were respectively 25.8 days (11.1), 12.9 days (13.4) and 9.2 days (9.3), showing a statistically significant difference between NST and AZM alone (p<0.0001) or AZM+HCQ (p<0.0001). The statistical difference between NST and AZM was confirmed (p=0.0149) as well as the difference with AZM+HCQ (p=0.0002). No cardiac toxicity was recorded in any patient. No statistical difference was shown between AZM and AZM+HCQ groups, although the dual therapy tended to be more effective in patients over 50 years, based on an analysis using the cox model.
\nConclusion: AZM and AZM+HCQ favourably impacted the course of the disease. We need trials, ideally prospective/double blind, to show if a statistical difference can be evidenced with a broader group, and clarify the indications of each treatment depending on initial clinical presentation.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Guérin",
"given": "Violaine",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lévy",
"given": "Pierre",
"sequence": "first"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Jean-Louis",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lardenois",
"given": "Thierry",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lacrosse",
"given": "Philippe",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sarrazin",
"given": "Emmanuel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Andreis",
"given": "Natacha Regensberg-de",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wonner",
"given": "Martine",
"sequence": "first"
}
],
"container-title": "Asian Journal of Medicine and Health",
"container-title-short": "AJMAH",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
20
]
],
"date-time": "2020-07-20T01:58:40Z",
"timestamp": 1595210320000
},
"deposited": {
"date-parts": [
[
2022,
10,
5
]
],
"date-time": "2022-10-05T07:10:25Z",
"timestamp": 1664953825000
},
"indexed": {
"date-parts": [
[
2024,
2,
8
]
],
"date-time": "2024-02-08T03:37:00Z",
"timestamp": 1707363420054
},
"is-referenced-by-count": 21,
"issued": {
"date-parts": [
[
2020,
7,
15
]
]
},
"link": [
{
"URL": "https://www.journalajmah.com/index.php/AJMAH/article/download/30224/56706",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.journalajmah.com/index.php/AJMAH/article/download/30224/56707",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.journalajmah.com/index.php/AJMAH/article/download/30224/56706",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4694",
"original-title": [],
"page": "45-55",
"prefix": "10.9734",
"published": {
"date-parts": [
[
2020,
7,
15
]
]
},
"published-online": {
"date-parts": [
[
2020,
7,
15
]
]
},
"publisher": "Sciencedomain International",
"reference-count": 0,
"references-count": 0,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.20944/preprints202005.0486.v1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://journalajmah.com/index.php/AJMAH/article/view/431"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Environmental Engineering"
],
"subtitle": [],
"title": "Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19",
"type": "journal-article"
}
